Advancing ADC Therapeutics in Oncology
Expert Support for ADC Drug Development, Driving Success from Lab to Clinic
TD2 is a scientifically driven CRO focused on the development of novel cancer treatments including the development of antibody drug conjugate (ADC) therapies in oncology. Our comprehensive range of services includes preclinical testing, regulatory compliance assistance, and management of early phase clinical trials. TD2 equips biotech and pharmaceutical companies with the necessary tools and expertise to advance their ADC therapeutic candidates from initial discovery to clinical evaluation. Utilizing advanced technologies and our collaborative approach, we ensure that our partners meet critical milestones in their drug development process. TD2 is strategically positioned to support the progression of innovative ADC therapies, aiming to improve treatment outcomes and enhance patient quality of life.
Features and Benefits:
- Translational services to support development from the preclinical stage through early phase clinical trials
- Experienced drug development team with deep clinical and scientific knowledge
- Regulatory and Toxicology Experts in ADC Therapeutics
Request more information about our ADC Evaluation Services
Preclinical Services:
TD2 offers specialized preclinical services for advancing ADC therapies in oncology, covering both in vitro and in vivo studies. Our in vitro services include comprehensive assays for characterizing ADCs, such as antigen expression, binding studies, and cytotoxicity assessments. In vivo services include pharmacology studies using over 300 cancer models and advanced imaging techniques to optimize ADC development. This integrated approach ensures robust support for translating ADC research from the lab to clinical trials.
![]()
In Vitro
Characterization
Protein expression via western blotx
ADC Binding via flow cytometryx
Gene and protein expression via flow and qPCRx
In vitro activity assays by colony formation assay (CFA)x
Tumor penetration assaysx
Flow analysis of cytotoxicity, DNA damage, cell cycle and apoptosisx
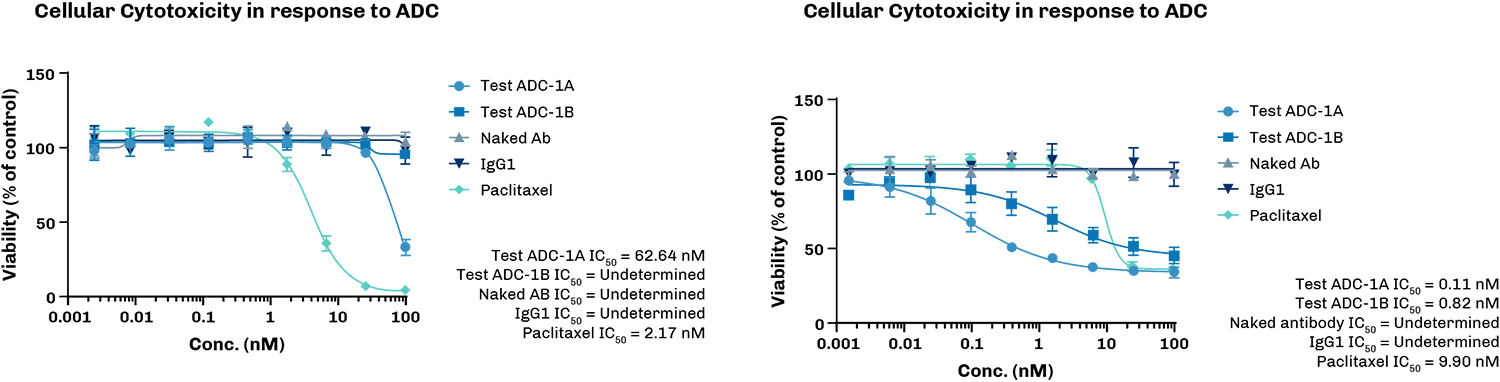
Determination of cellular toxicity in response to ADCx
Full PK evaluation services
Payload analysis from Plasma Stability analysis
![]()
In Vivo
Pharmacology
In vivo efficacy studiesx
Specialty surgical & orthotopic modelsx
Over 300+ CDX modelsx
Optical Imaging
Additional Resources
Preclinical
Top Trends in ADC Drug Development
Regulatory, Preclinical
Navigating the Preclinical-to-Clinical Transition for ADCs: Regulatory & Study Design Strategies
Regulatory Support:
TD2 offers comprehensive regulatory support to expedite the development of Antibody Drug Conjugates (ADCs) for cancer therapies. With a team skilled in oncology-focused medical writing, regulatory expertise in Chemistry, Manufacturing, and Controls (CMC), as well as pharmacology and toxicology, TD2 provides a tailored approach to navigate complex regulatory landscapes. Our services encompass strategic consultation, program management, and direct FDA liaison activities, ensuring that each step of the ADC development process aligns with regulatory requirements to accelerate time to market.
Clinical Trial Management Services:
TD2’s clinical study management services are designed to support the advancement of ADC cancer treatments through the clinical trial phase. TD2 offers a comprehensive portfolio program management including study design, patient recruitment, data management, and site engagement. Our approach utilizes advanced technology and personalized strategies to ensure efficient trial progression. TD2 also focuses on robust program analysis and strategic consultation to navigate complex regulatory requirements, aiming to optimize trial outcomes and speed up the introduction of new cancer therapies to the market.
![]()
Comprehensive
Clinical Trial Services Include:
- Study design & protocol development
- Patient identification and site engagement
- Site management and monitoring
- Third-party vendor management
- Biostatistics
- Data management
- Clinical Flow Cytometry
- Pharmacovigilance
- Medical monitoring
- eTMF management
- Quality Assurance
GET STARTED
Work with a team who believes in your research as much as you do.
Are you ready to start your ADC therapy studies? Partner with a collaborative oncology CRO that believes in your treatment as much as you do. Take the first step today and contact our experts.
Protein expression via western blot
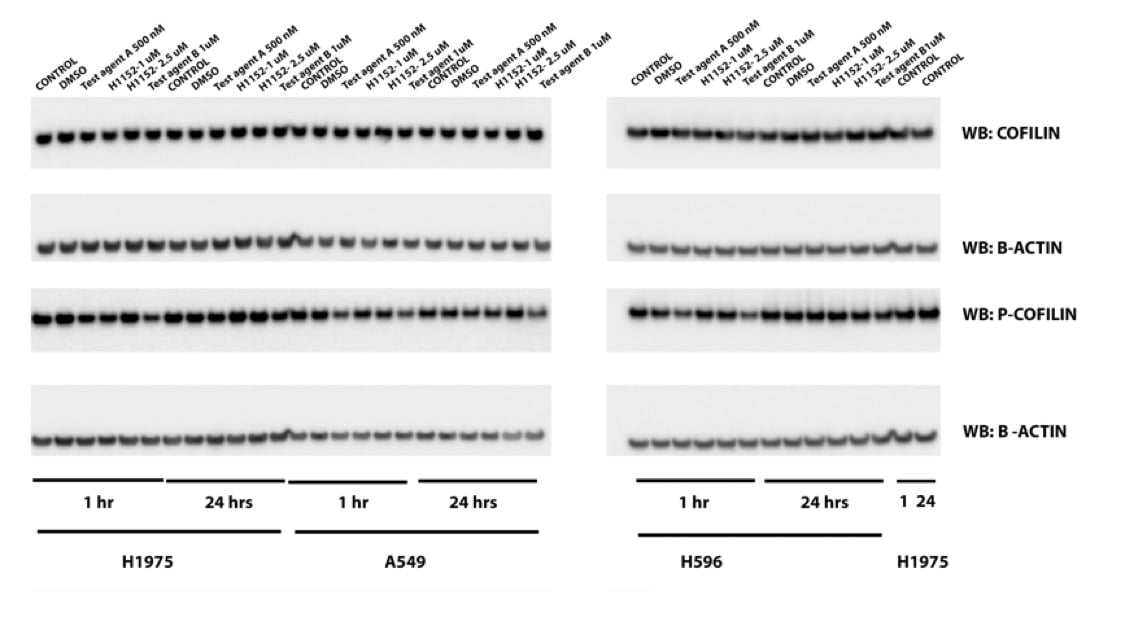
Changes in phosphorylation of Cofillin in three cancer cell lines
ADC Binding via flow cytometry
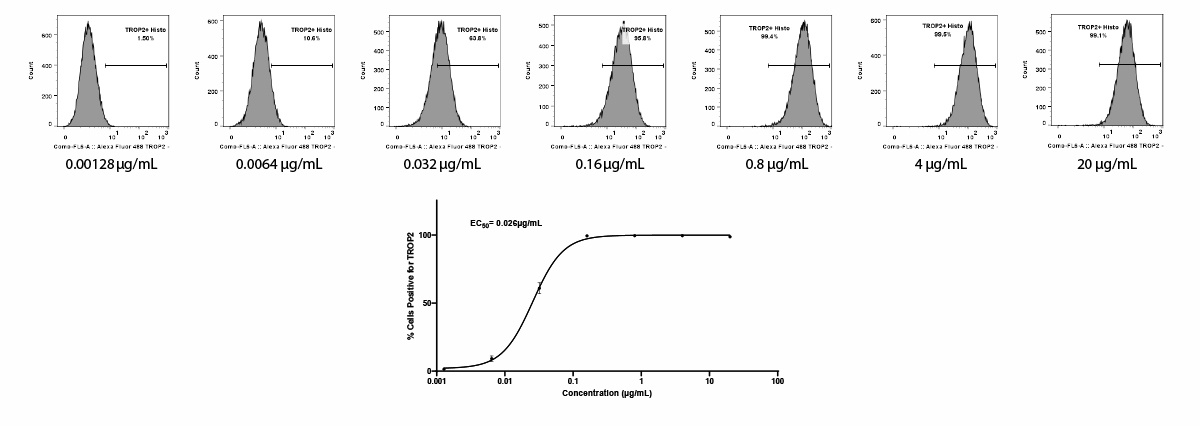
Sacituzumab binding affinity and EC50 determination
Gene and protein expression via flow and qPCR

In vitro activity assays by colony formation assay (CFA)

Tumor penetration assays

Flow analysis of cytotoxicity, DNA damanage, cell cycle and apoptosis
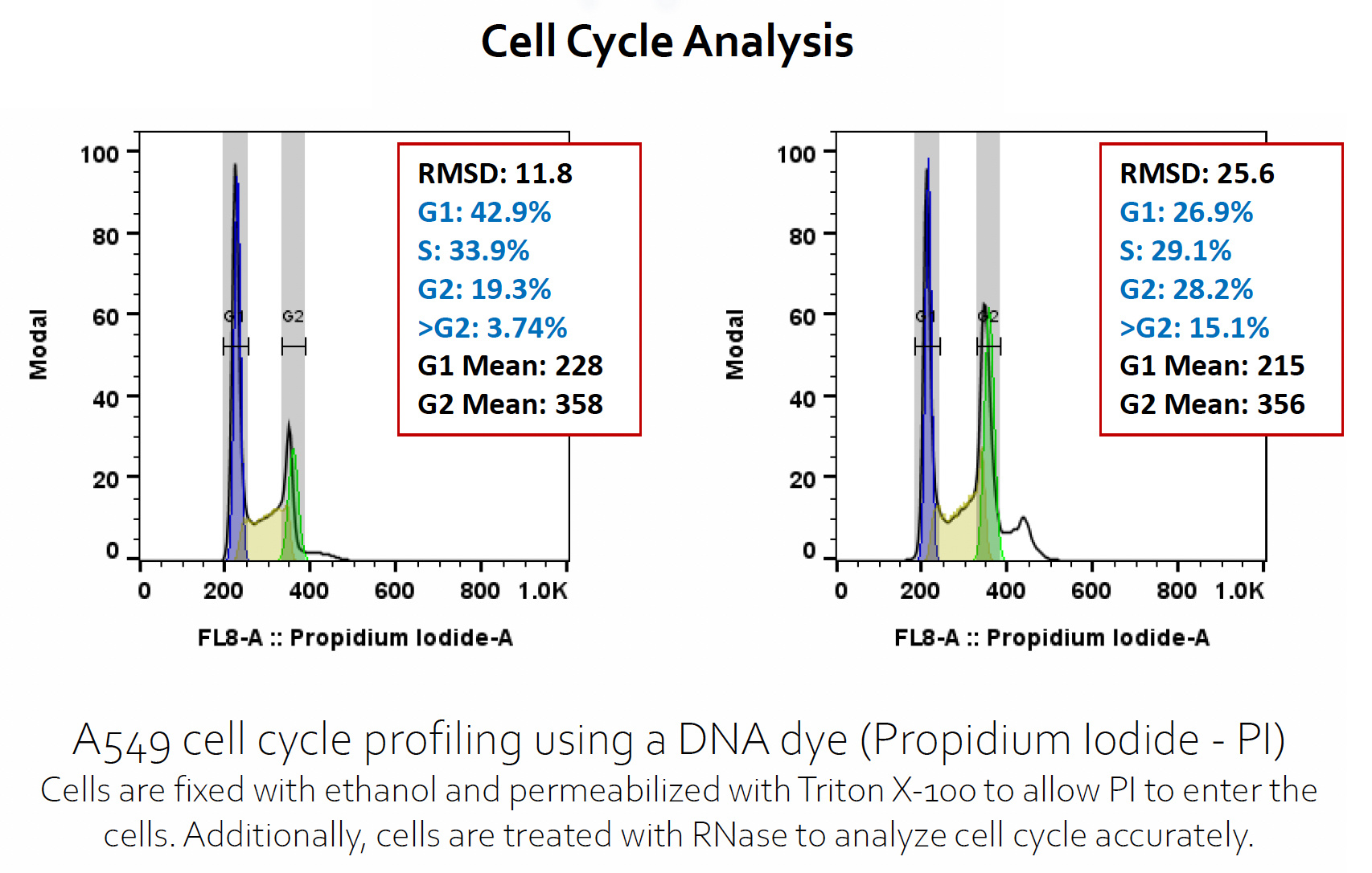
Determination of cellular toxicity in response to ADC
In vivo efficacy studies
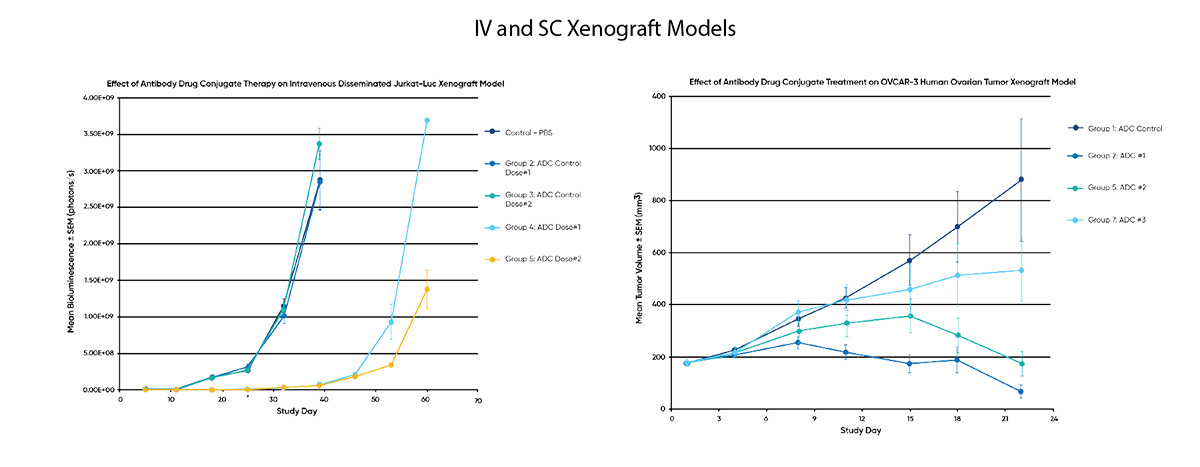
Specialty surgical & orthotopic models
|
Xenograft Lines |
|
|---|---|
| Intracranial | BT-142, BT-474-luc, U251, U251-luc, U87-MG, U87-MG-luc, NCI-H1975-luc, GMB-PDX |
| Intrahepatic | Hep3B, Huh-7-luc |
| Intrapancreatic | BxPC3, BxPC3-luc, AsPC-1, AsPC-1-fluc, Panc-1-luc, HPAF-II-luc |
| Intrathoracic | NCI-H460-luc |
| IP Disseminated | HCT-116-luc, SK-OV-3-luc, Cal-27-fluc, OVCAR-8 |
| Mammary Fat Pad | BT-474, HCC70, MDA-MB-231, MDA-MB-231-luc |
| Ovarian Ascites | A2780, A2780CP, ES-2, OVCAR-8 |








