Flow Cytometry FAQ
Flow cytometry is a key tool in drug development, offering detailed insights into how drugs interact with cells, helping to identify biomarkers, and detecting potential safety issues. With over 20 years of experience, TD2 provides custom assay development and validation, supporting both nonclinical and clinical studies. This FAQ covers common questions about how flow cytometry is used in drug development and explains TD2's services, including assay customization, specimen handling, and data reporting, to support every stage of the process.
Why use flow cytometry for drug development?
Flow cytometry is a powerful tool in drug development for several reasons. These include:
- High throughput analysis allows for the rapid analysis of thousands of cells per second, making it ideal for screening large libraries of compounds.
- Flow cytometry can measure multiple parameters simultaneously, such as cell size, granularity, and the expression of various markers. This helps in understanding the complex interactions between drugs and cellular targets.
- Biomarker identification allows for identifying and evaluation of biomarkers, which are crucial for understanding disease mechanisms and the effects of drugs.
- For pharmacokinetics and pharmacodynamics (PK/PD), Flow cytometry helps in studying the relationship between drug concentration and its effect on cells, providing insights into the drug’s efficacy and safety.
- Flow cytometry can detect subtle changes in cell populations, helping to identify potential toxic effects of new drugs early in the development process.
Overall, flow cytometry’s ability to provide data on heterogeneous cell populations makes it an invaluable tool in the drug development pipeline.
What stages of drug development does TD2 support?
TD2 operates as a contract research organization (CRO) offering flow cytometry-based assays for nonclinical studies at both Scottsdale and Bothell sites. The Bothell site offers GLP and non-GLP services as well as support for clinical trials.
What is TD2 expertise in flow cytometry?
TD2’s most experienced flow cytometry scientists have 20+ years working in the field. The flow cytometry staff are skilled in assay development/qualification and are at the forefront for determining the criteria for flow cytometry assay and instrument validation.
What flow cytometry platforms/instruments and how many parameters can be tested at TD2?
- Three BD FACSCanto II systems with two scatter parameters and up to 8 fluorescent channels
- Four BD FACSCanto systems with two scatter parameters and up to 10 fluorescent channels
- Two Cytek Aurora spectral flow cytometers with two scatter parameters and up to 26 fluorescent channels
- One Miltenyi MACS Quant analyzer with two scatter parameters and up to 14 fluorescent channels
What types of flow cytometry assays can TD2 perform?
TD2 offers an integrated platform for nonclinical and clinical drug development based on expertise in:
- Immunophenotyping
- T-lymphocyte, B-lymphocyte and Natural Killer cells (TBNK)
- T Regulatory Cells
- T Memory, Naïve and Effector cells
- T Cell Activation
- B Memory and Plasma cells
- Humanized mice panels
- Custom panels are also available
- Intracellular Protein Analysis
- Intracellular Cytokines
- Phosphorylated Proteins
- Cellular Functional Assays
- NK Cell Function
- Basophil Activation Test (BAT)
- Cell Cycle and Apoptosis
- Receptor Occupancy
- PBMC Services
- Custom flow cytometry panels and assays available upon request
The technology of flow cytometry also provides invaluable support to the preclinical animal models available at the TD2 Scottsdale facility.
Is it possible for TD2 to replicate a flow assay from a publication?
Yes, TD2 can develop assays using literature established protocols for panel design, staining procedures and instrument settings. Our scientists have the knowledge and experience to optimize the conditions for our environment and instrumentation.
How should specimens be collected and transported for flow cytometry assays?
Specimen Types
- Blood: Generally collected in K2EDTA (lavender top) or Sodium Heparin (green top) tubes.
- Bone Marrow: Generally collected in K2EDTA.
- Tissue: Fresh tissue samples should be kept in a sterile container with a suitable transport medium.
- Stability tubes or media can be used for increased stability needs.
Collection and Shipping Guidelines
- Aseptic technique should be used to avoid contamination.
- Adequate volume of the specimen needs to be collected per tube size. Typically 1-3 mL for blood and bone marrow.
- Labeling of all specimens with identifying information and collection date/time should be followed.
- Specimens should be transported at an appropriate temperature with the proper packaging to preserve specimen integrity and containment and are processed within stability guidelines.
Do you offer standard or off-the-shelf panels? How much work is required to modify an existing
antibody panel?
At TD2, we pride ourselves on being able to customize our antibody panels to meet our client’s needs, so we have a limited number of pre-qualified/validated antibody panels. If working with an established antibody panel, TD2 scientists follow best practices for the re-qualification/validation once a flow cytometric method has been modified. This includes an assessment of the impact of different types of assay modifications on assay performance. Recommendations regarding which parameters to evaluate depending on the type of modification and the impact of assay modification on the assay's intended use will be determined. Timelines will depend on the extent of modification and how much troubleshooting is required.
What is your capacity and how soon can you take on new projects?
Currently, the flow cytometry laboratories at both the Scottsdale and Bothell sites have sufficient capacity to start new projects within 1-2 weeks after contracts are signed, depending on reagent availability without backorders.
Can we get raw flow data? How long from running the sample to delivery of the data?
Yes, TD2 can provide the raw .fcs files via ShareFile or via client specific portals for clients to perform their own analysis. These files are typically available within one week of sample collection.
What are the final deliverables of the flow cytometry portion of the project? What are the typical timelines?
Depending on project scope, TD2 can provide the flow cytometry data in MS Excel tables, graphical representations of the data or a flow cytometry report, either a summary report or a more comprehensive report with detailed analysis. General timelines for each are as follows: Data Tables within 1-2 weeks post collection or monthly for clinical flow projects, Graphing within 2-3 weeks post collection, Summary Report within 2-3 weeks post study end, and Comprehensive Report within 3-4 weeks post study end. These target timelines depend on the deliverables requested and can be impacted by the complexity of the flow cytometry services being performed.
Is there an opportunity for co-authorship of posters/papers between sponsors and TD2?
TD2 scientists look forward to exploring potential ways to collaborate with our clients for developing novel flow cytometry assays or discovering novel uses for existing flow cytometry technology leading to potential co-authorship on posters, presentations or manuscripts.
Can we visit either lab while samples are being processed?
TD2 Scottsdale and Bothell sites welcome the opportunity to host our clients to observe sample processing, conduct on-site audits or just to visit as a part of due diligence. Please contact us to set up an agreed upon time.
Request more information about our Flow Cytometry Services
Contact our experts to help advance your drug development with TD2's trusted Flow Cytometry Services.
GET STARTED
Work with a team who believes in your research as much as you do.
Are you ready to start your Flow Cytometry studies? Partner with a collaborative oncology CRO that believes in your treatment as much as you do. Take the first step today and contact our experts.
![]() Receptor Occupancy
Receptor Occupancy
Binding of fluorophore labeled “drug” in relation to decreasing dose of unlabeled “drug”

![]() Receptor Occupancy
Receptor Occupancy
![]() PBMC Services
PBMC Services
Evaluate responses of PBMCs by profiling the function/phenotype of subpopulations

![]() Immune Population Phenotyping
Immune Population Phenotyping
Immune cell population analysis of subcutaneous CT26 murine colon carcinoma tumors
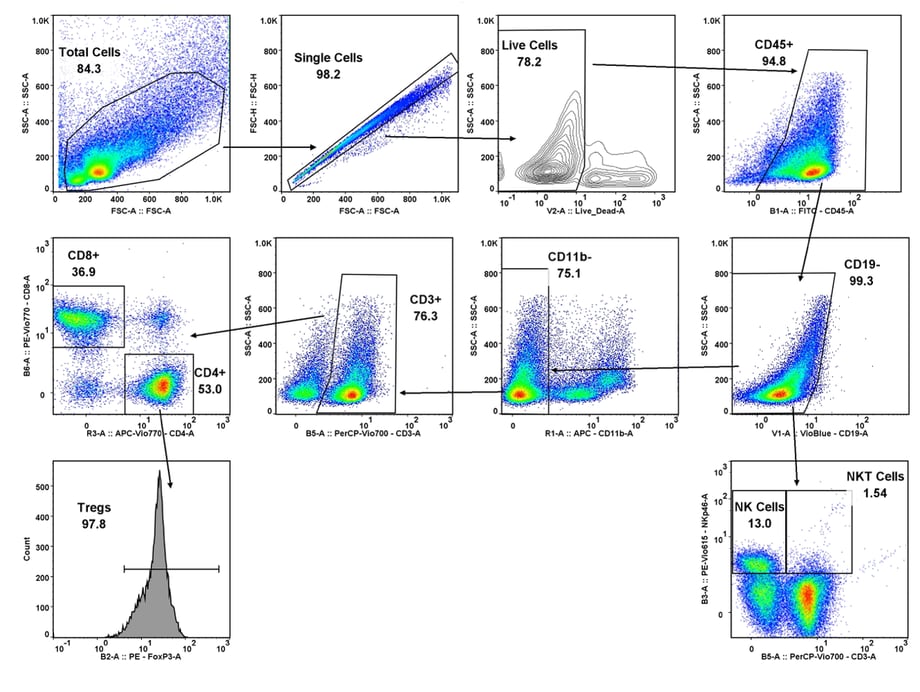
CT26 SC tumors: Gating strategy T cells and NK cells.
Gating involved identifying the total cell population, and then gate out the doublets, gate out the dead cells, move on to our first marker CD45 which gates out any non-immune cells. The bottom right cytogram shows the different NK cell populations. The far left middle cytogram depicts the two T cell population CD4+ and CD8+. Values are % of total CD45+ cells.
![]() Cancer Cells/Tumor Specific Marker Expression
Cancer Cells/Tumor Specific Marker Expression
Cancer cells/tumors specific markers expression
- Viability dye inclusion
- Cell lines and single-cell suspension tumors
- Surface staining

![]() Fluorescent Proteins Expression
Fluorescent Proteins Expression
Fluorescent proteins expression
- Viability dye inclusion
- Cell lines and transfected primary cells
- Surface staining

![]() Cell Cycle Analysis
Cell Cycle Analysis
Cell cycle analysis A549 cells
- Propidium iodide
- Cell lines

![]() Intracellular Protein Analysis
Intracellular Protein Analysis
- Intracellular Cytokines
- Phosphorylated Proteins
- T-Regs
- Phosphoroteins
- Cytokines

![]() Cellular Functional Assays
Cellular Functional Assays
- NK Cell Function
- Basophil Activation Test (BAT)



