clinical research
solutions
Your Oncology CRO Partner
Delivering Tailored Clinical Trial Services for Breakthrough Therapies
Your oncology treatment’s success starts with the right partner. At TD2 Oncology, we provide comprehensive clinical research solutions tailored specifically for oncology. From trial design and feasibility through site management, data analysis, and final reporting, our integrated team ensures every aspect of your study is executed with precision and care.
Through each milestone, our experienced project managers and oncology experts collaborate with you to navigate challenges, identify opportunities, and adapt strategies for optimal outcomes. We prioritize advanced technology and personalized solutions to connect the right patients with the right trials, ensuring progress without compromise.
As a full-service oncology CRO, TD2 Oncology positions your therapy for regulatory and clinical advancement while empowering your team to focus on innovation. Let us help you transform potential into progress.
Explore Our Clinical Trial Services
Explore Our Clinical Trial Services
Not your typical CRO
What sets TD2 apart in oncology clinical trial management?
- Oncology-Focused Expertise: Our sole focus on oncology trials gives us unparalleled insight into the complexities of cancer research.
- Integrated Ecosystem Approach: As part of the TD2 Oncology Ecosystem, we bring together regulatory, clinical, and scientific expertise for seamless drug development.
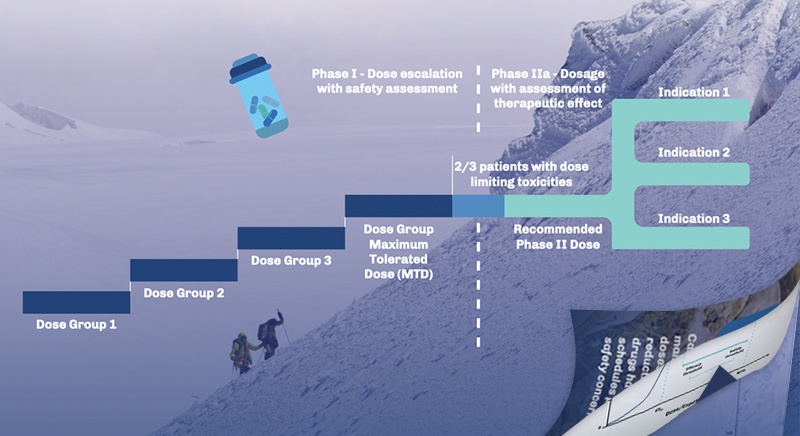
- Accelerated Start-Up Timelines: Our unique Clinical Trial Enablement program is designed to streamline processes and optimize resource allocation from IND submission to the enrollment of the first patient
- Customized Trial Design: We tailor each study to your therapeutic and operational needs, ensuring precision and adaptability.
- Dedicated Team: A team of oncology specialists committed to providing personalized support throughout your trial.
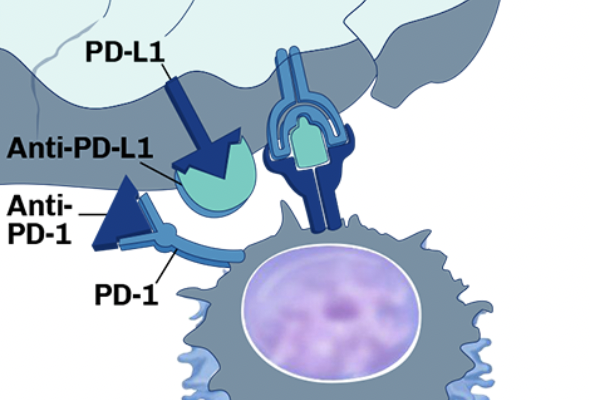
- Clinical Flow Cytometry Expertise: We deliver rapid, high-quality analysis to evaluate pharmacodynamic effects, immune responses, and biomarker expression to support your studies and accelerate decision-making during clinical trials.
Driving Results Through Data
Clinical Trial Performance Metrics

Therapeutic Spotlight
Additional Resources
The TD2 Oncology Ecosystem
Seamless Integration from Preclinical to Clinical Success
TD2 Oncology offers a comprehensive suite of study management services, ensuring a seamless and efficient clinical trial process from preclinical stages through regulatory approvals and beyond. Our integrated approach includes:
- Preclinical Services: Leveraging innovative technology and experienced scientists to deliver robust preclinical data which guide your clinical strategy.
- Regulatory Support: Guiding you through the complex regulatory landscape with expert advice and documentation support for an expedited path into the clinic.
- Clinical Trial Management: Providing end-to-end management of your clinical trials, from site selection and patient recruitment to data management and analysis ensuring a streamlined clinical experience.
By partnering with TD2 Oncology, you benefit from a streamlined process that minimizes delays and optimizes resource allocation, ensuring that your trial progresses smoothly at every stage. Our commitment to quality and efficiency means you can focus on your research, while we handle the operational complexities.
GET STARTED
Put your clinical trial in the hands of a team who believes in your research as much as you do.
Are you ready to start your preclinical trial? Partner with a collaborative oncology CRO that believes in your treatment as much as you do. Take the first step today and contact our experts.