Humanized Mouse Models for In vivo Immunotherapy Evaluation
PBMC, CD34+, and GEM Models
At TD2, we specialize in providing comprehensive in vivo studies for the evaluation of immunotherapies using advanced humanized mouse models. Our portfolio includes PBMCs, CD34+, and humanized immune checkpoint inhibitor GEM models, offering robust platforms to assess the efficacy and safety of novel immuno-oncology drugs. These models closely mimic human immune responses, enabling precise preclinical evaluations and accelerating the development of effective immunotherapy treatments. Additionally, our on-site flow cytometry capabilities ensure the accurate engraftment of human immune cells.
Features and Benefits:
- Accurate Human Immune Response Simulation: Our humanized mouse models, including PBMC, CD34+, and humanized immune checkpoint GEM models, closely replicate human immune system interactions, providing reliable insights into therapy efficacy.
- Enhanced Predictability: These models offer superior predictive power for clinical outcomes, bridging the gap between preclinical studies and human clinical trials.
- Diverse Immune Contexts: With a range of models available, we can tailor studies to various immune contexts, ensuring comprehensive evaluation of immune-oncology drugs.
- Accelerated Drug Development: Utilizing these advanced models accelerates the drug development process by providing early and accurate data on therapeutic potential and safety.
- Customized Study Design: TD2 offers bespoke study designs to meet specific research needs, leveraging our expertise and advanced models to optimize preclinical evaluations.
Request more information about our humanized mouse models
Contact our experts to help advance your immunotherapy candidate with our trusted preclinical research services.
PBMC Humanized Mouse Models
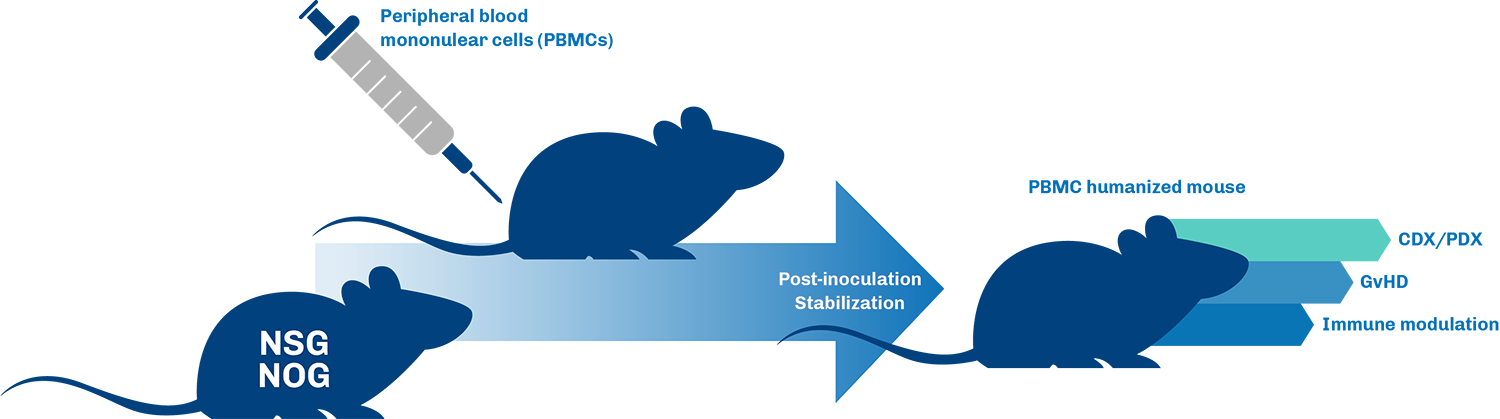
Benefits of using PBMC mice for I/O research
- Human Immune Cell Integration: PBMC models incorporate human peripheral blood mononuclear cells, allowing researchers to study interactions between human immune cells and cancer cells in a controlled in vivo environment, leading to more relevant preclinical data.
- Immune Checkpoint Inhibition Evaluation: These models are ideal for evaluating the efficacy of immune checkpoint inhibitors and other immunotherapies, providing critical insights into their mechanisms of action and potential clinical efficacy.
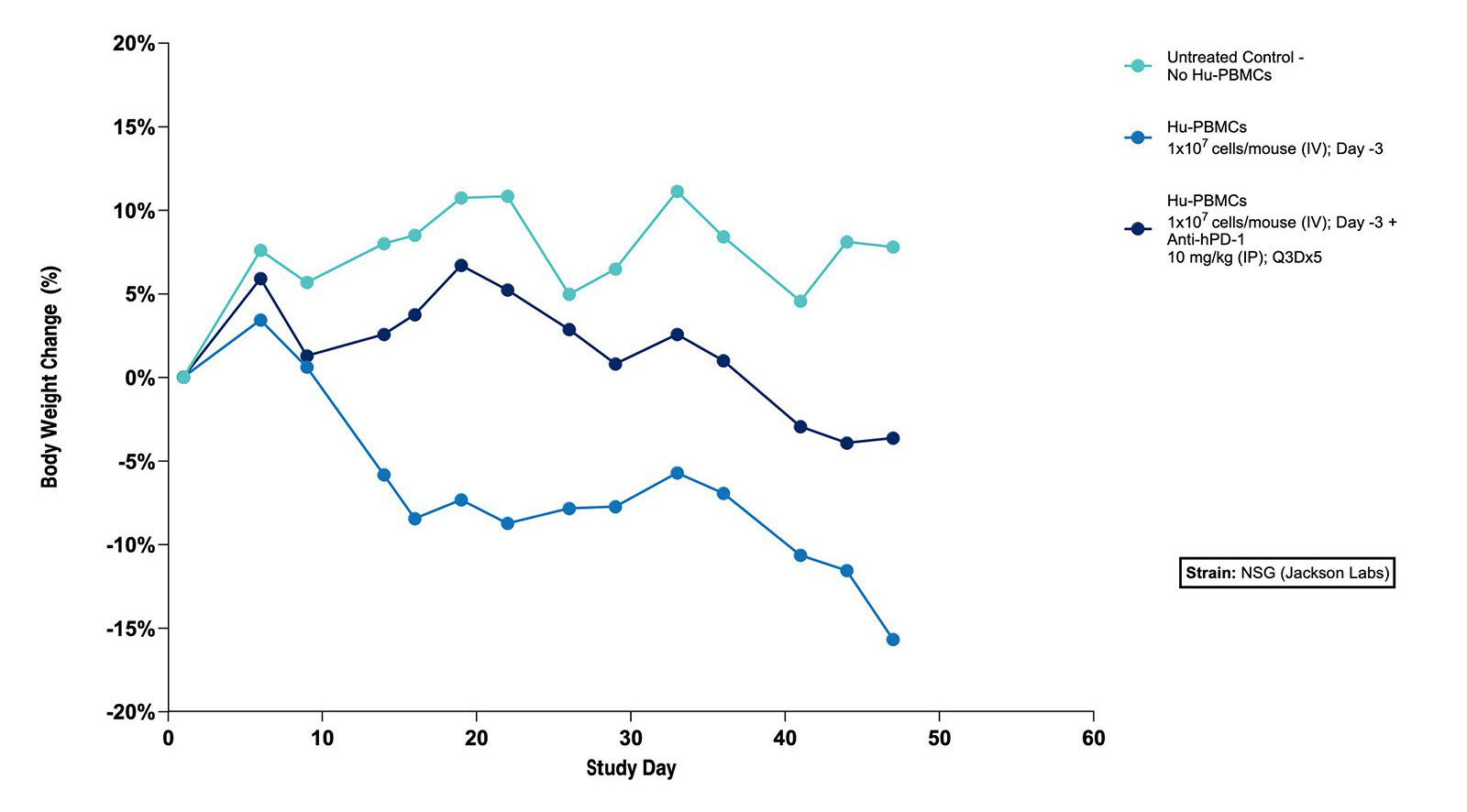 Fig 1. Effect of Hu-PBMCs Alone and in Combination with Anti-hPD-1 on Body Weight in the Subcutaneous MKN45 Human Gastric Tumor Xenograft Model (N=5 mice/group) |
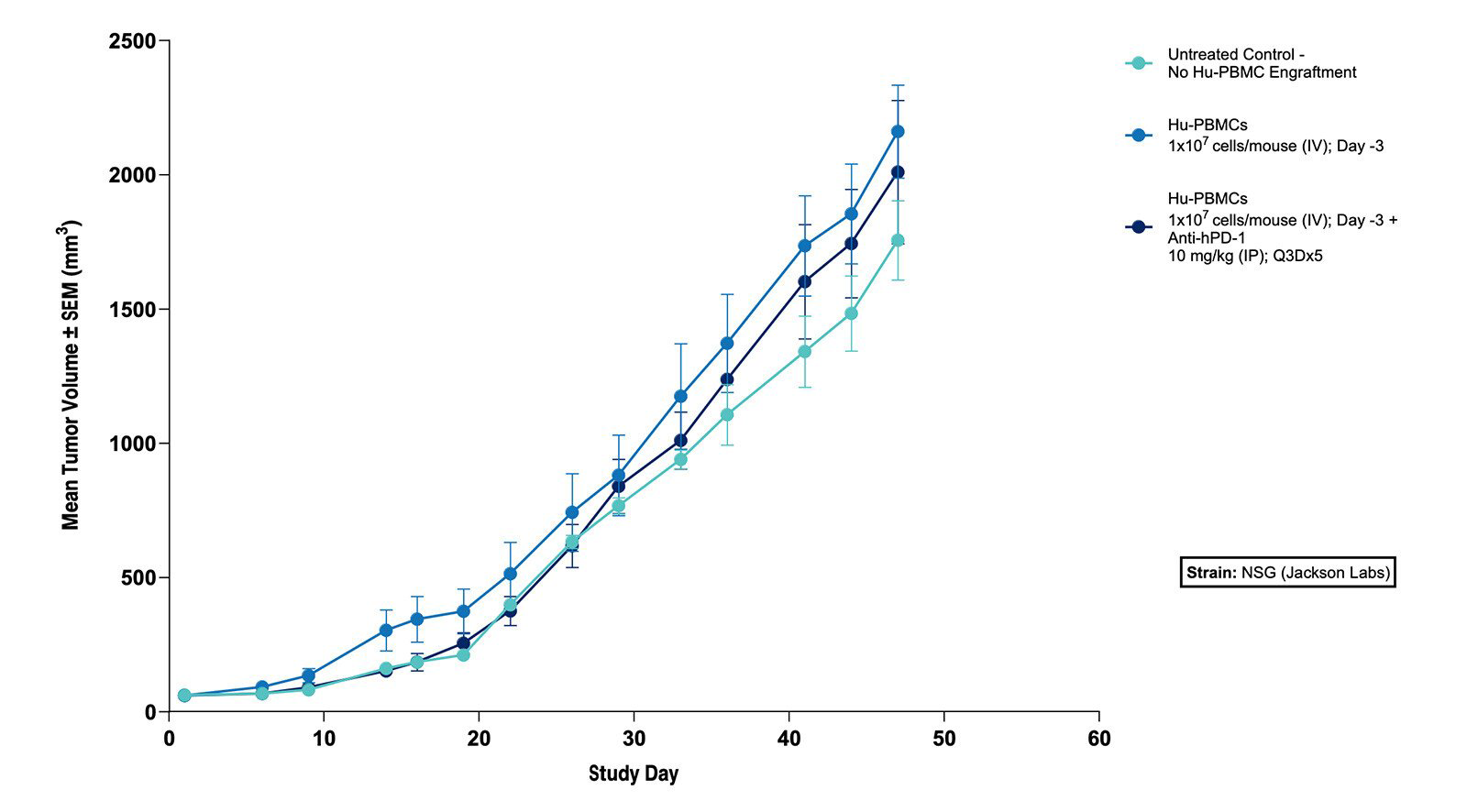 Fig 2. Effect of Hu-PBMCs Alone and in Combination with Anti-hPD-1 on Tumor Volume in the Subcutaneous MKN45 Human Gastric Tumor Xenograft Model (N=5 mice/group) |
 Fig 1. Effect of Hu-PBMCs Alone and in Combination with Anti-hPD-1 on Body Weight in the Subcutaneous MKN45 Human Gastric Tumor Xenograft Model (N=5 mice/group)  Fig 2. Effect of Hu-PBMCs Alone and in Combination with Anti-hPD-1 on Tumor Volume in the Subcutaneous MKN45 Human Gastric Tumor Xenograft Model (N=5 mice/group) |
CD34+ Humanized Mouse Models

Benefits of using CD34+ mice for I/O research
- Long-Term Human Immune System Development: CD34+ mouse models are engrafted with human hematopoietic stem cells, leading to the development of a long-term, self-renewing human immune system. This allows for extended studies on immune response and therapy effects over time.
- Multifaceted Immuno-Oncology Research: These models support the study of a wide range of immuno-oncology treatments, including cell therapies, vaccines, and antibody-based therapies, providing a versatile platform for preclinical research.
- High Relevance to Human Hematopoiesis: CD34+ models closely mimic human hematopoiesis and immune cell differentiation, making them highly relevant for studying hematologic malignancies and evaluating hematopoietic toxicities of new immunotherapies.
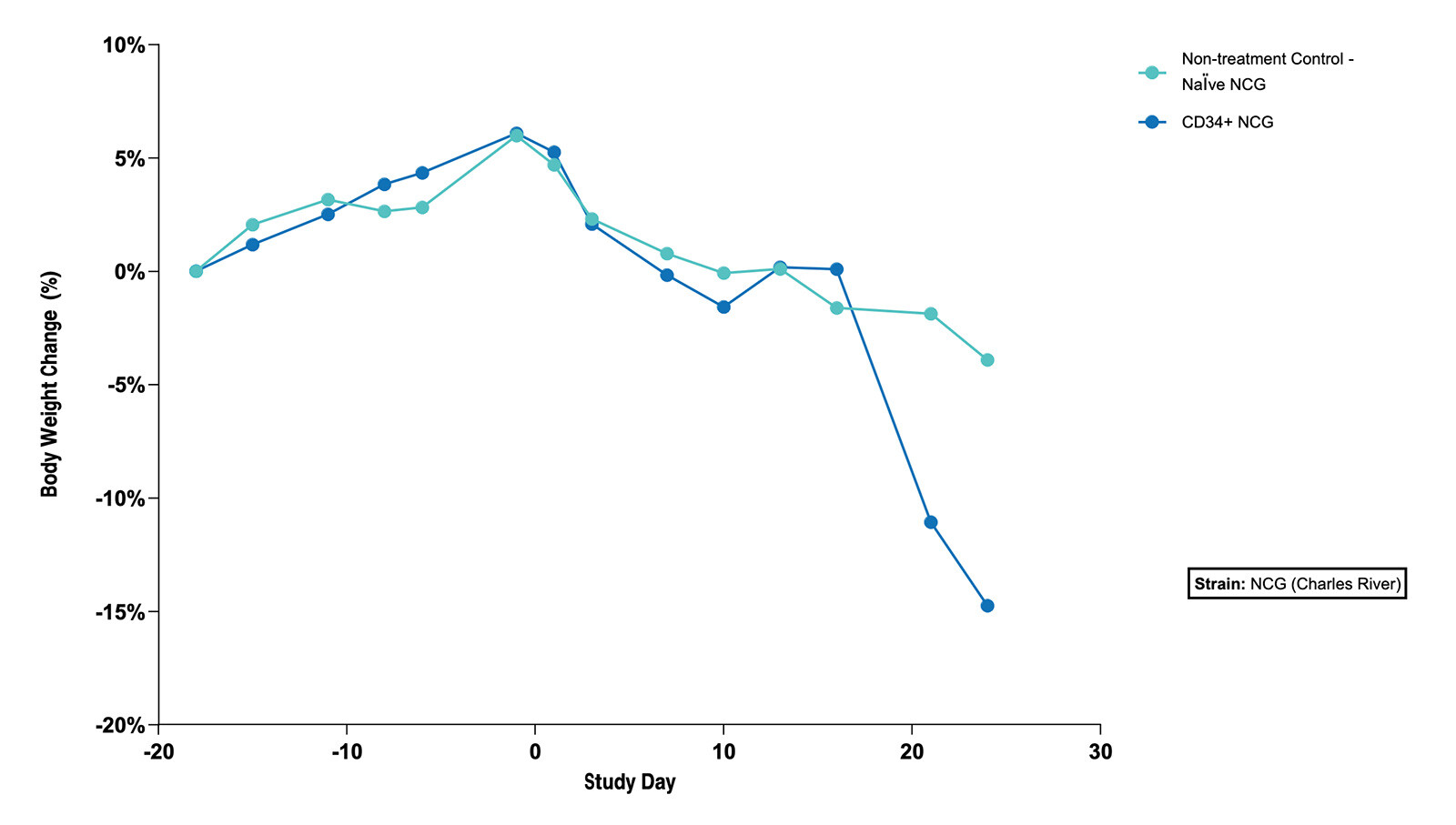 Fig 3. The Subcutaneous HT1376 Human Bladder Tumor Xenograft Model in CD34+ and Naive NCG Mice - % Body Weight Change (N=5 mice/group) |
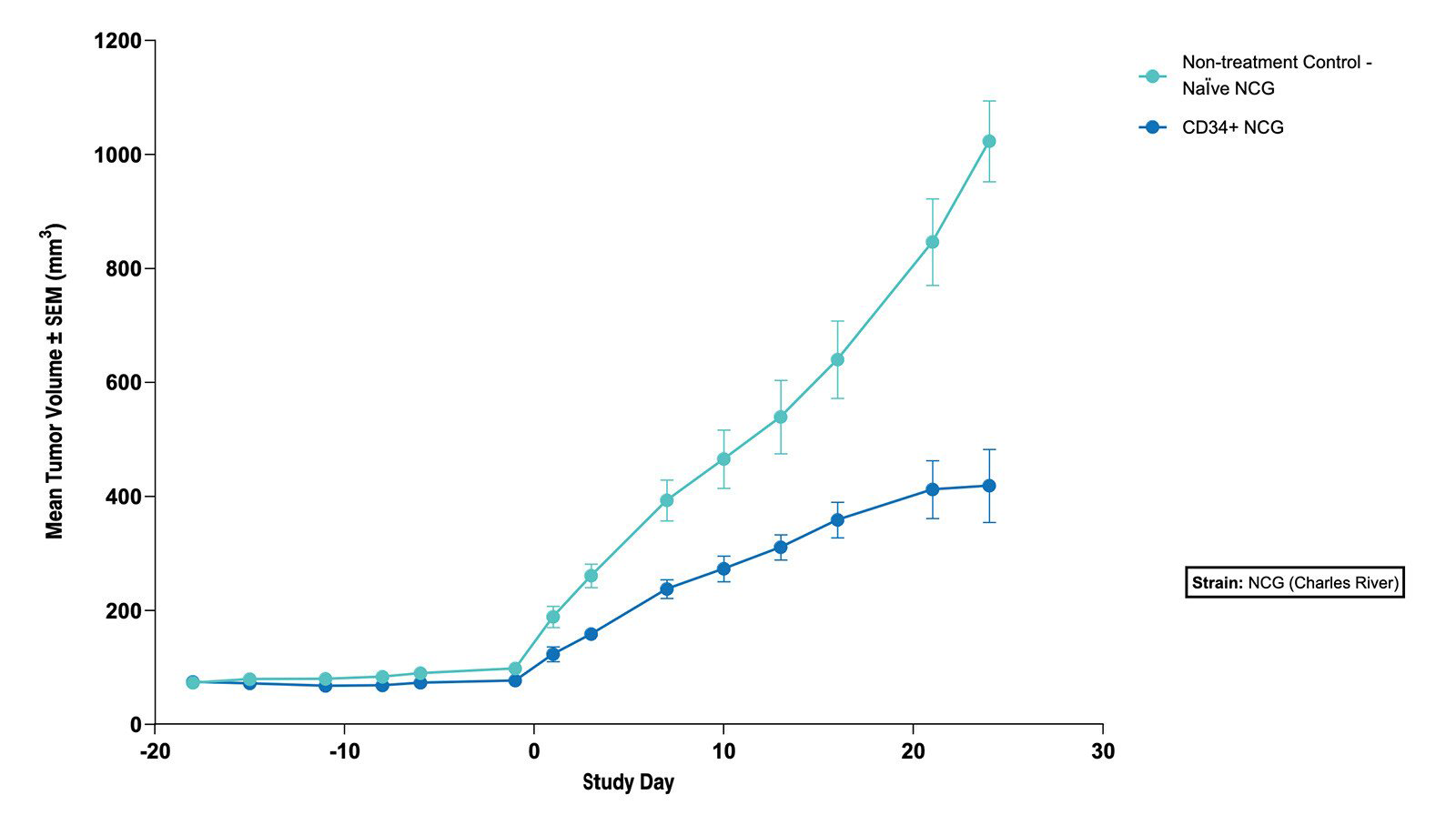 Fig 4. The Subcutaneous HT1376 Human Bladder Tumor Xenograft Model in CD34+ and Naive NCG Mice - Tumor Growth Curve (N=5 mice/group) |
 Fig 3. The Subcutaneous HT1376 Human Bladder Tumor Xenograft Model in CD34+ and Naive NCG Mice - % Body Weight Change (N=5 mice/group)  Fig 4. The Subcutaneous HT1376 Human Bladder Tumor Xenograft Model in CD34+ and Naive NCG Mice - Tumor Growth Curve (N=5 mice/group) |
GEM Humanized Immune Checkpoint Inhibitor Models
Benefits of using humanized immune checkpoint inhibitors for I/O research
- Enable the evaluation of human-specific biological therapies in vivo faster than traditional animal models
- Double KI models allow combination therapy evaluation
- Lack of expression of the murine target gene, avoiding cross-reactivity
- Provides a more predictive model for how drugs will perform in the clinic
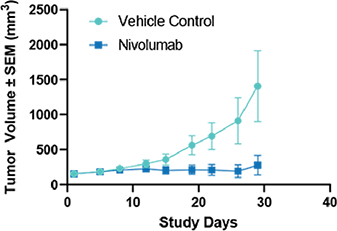 Fig 5. Humanized PD-1 mice (hPD-1) were inoculated with MC-38 cells expressing human PD-L1 (hPD-L1). Mice were treated with Nivolumab (10 mg /kg) OD 3 x 4 Results are shown as TV + /- SEM |
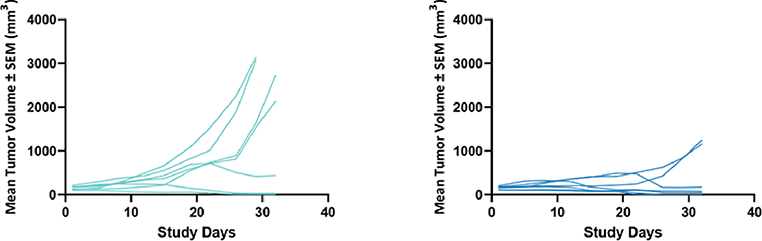 Fig 6. Individual spider plots of humanized PD1 mice (hD1) inoculated with MC-38 cells expressing human PD-L1 (hPD-L1) Mice were treated with vehicle (left) or Nivolumab (10 mg/kg) QD 3 x 4 (right) |
 Fig 5. Humanized PD-1 mice (hPD-1) were inoculated with MC-38 cells expressing human PD-L1 (hPD-L1). Mice were treated with Nivolumab (10 mg /kg) OD 3 x 4 Results are shown as TV + /- SEM  Fig 6. Individual spider plots of humanized PD1 mice (hD1) inoculated with MC-38 cells expressing human PD-L1 (hPD-L1) Mice were treated with vehicle (left) or Nivolumab (10 mg/kg) QD 3 x 4 (right) |
Commonly Utilized Humanized Immune Checkpoint Inhibitor Models
(Contact Us for Full Model List)
| Single Target | Double Target | Triple Target | |
|---|---|---|---|
| hACE2 | hTREM2 | hCD47/hSIRPα | hFcgRs/hCD47/hSIRPα |
| hCD3E | hCD22 | hPD1/hLAG3 | hPD1/hPDL1/hCD137 |
| hHRAS | hCTLA4 | hPD1/hBTLA | hPD1/hPDL1/hCD40 |
| hPD1 | hLAG3 | hPD1/hOX40 | hPD1/hPDL1/hCD73 |
| hTIM3 | hSIRPα | hPD1/hCD137 | hPD1/hPDL1/hLAG3 |
| hBTLA | hVISTA | hPD1/hPDL1 | hPD1/hPDL1/hOX40 |
| hCD3EDG | hCD27 | hPD1/hCD47 | hPD1/hPDL1/hSIRPα |
| hIFNAR1 | hDPP4 | hPD1/hSIRPα | hPD1/hPDL1/hTIGIT |
| hPDL1 | hLLRC32 | hPD1/hCSF1R | hPD1/hPDL1/hTIM3 |
| hTMPRSS2 | hSTING1 | hPD1/hTIGIT | hPD1/hPDL1hCTLA4 |
| hCCN1 | hVTCN1 | hPD1/hCTLA4 | |
| hCD47 | hCD28 | hPD1/hTIM3 | |
| hIL12RB1 | hFcgRs | hPD1/hGITR | |
| hPVRIG | hOX40 | hPDL1/hB7H3 | |
| hTNFR2 | hTIGIT | ||
| hCD137 | hCD38 | ||
| hCD73 | hFcRn | ||
| hIL12RB2 | hRANK | ||
| hSIGLEC15 | |||
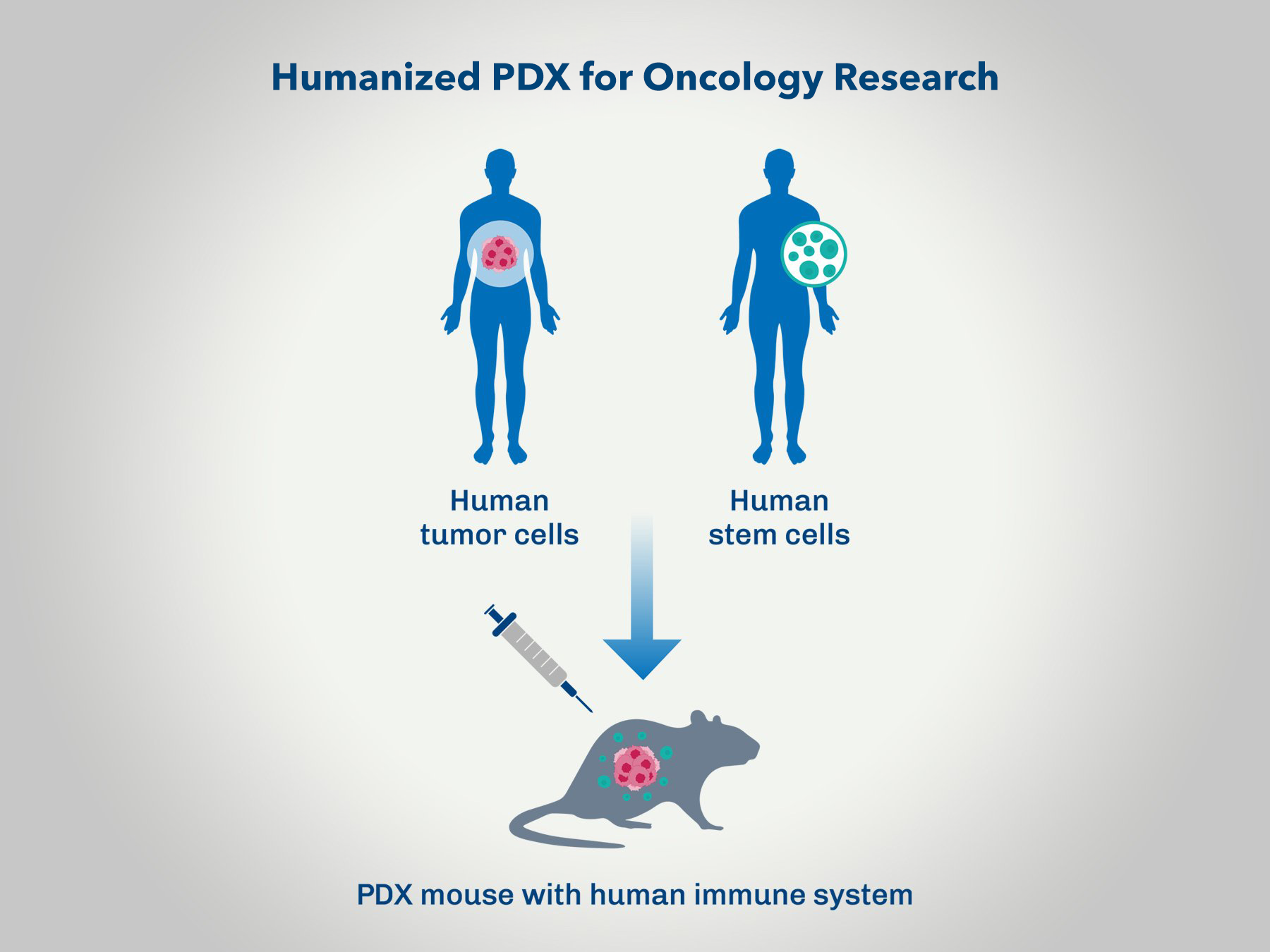
Humanized PDX Evaluation for Immuno-oncology
Conducting oncology research using Patient-Derived Xenograft (PDX) models with humanized mice offers a highly translational platform for assessing new cancer therapies. This approach combines the relevance of human tumor biology with an immune system that closely mimics human responses, enhancing the predictive power of preclinical studies. By leveraging humanized PDX models, researchers can gain valuable insights into the efficacy and mechanism of action of novel treatments in a setting that closely resembles the clinical scenario.
- Enhanced Predictive Value: Humanized PDX models provide a more accurate prediction of how human tumors will respond to new therapies.
- Immune System Relevance: These models incorporate a human immune system, allowing for the assessment of immuno-oncology therapies and their interactions with the tumor microenvironment.
- Personalized Medicine Insights: PDX models derived from individual patients' tumors can be used to study personalized treatment approaches, leading to more targeted and effective therapeutic strategies.

Immuno-oncology
Comprehensive preclinical services for immuno-oncology.
TD2’s extensive knowledge of CAR-T in both solid and hematologic tumors enables rapid evaluation of your CAR-T cell therapy, moving your therapeutic program forward to the clinic.
Large panel of human and murine tumor models
- 8 years of experience in cell therapy
- Expertise includes: CAR-T, NK-CAR, IPS-NKs, Macrophages
- Fully characterized for gene expression, TIL baseline populations, and response to common immune checkpoint inhibitors
Additional Resources
GET STARTED
TD2 navigates the complexities of drug discovery to guide you down a smarter path for earlier, more complete results.
Contact us today to learn more about our humanized mouse studies and how we can set your treatment up for success from the start.