Preclinical
In Vivo and In Vitro Services
to Advance Your Drug Discovery
TD2’s integrated suite of preclinical services provides our clients with data that drives the development of clinical strategy. From the discovery stage to NDA strategy, we offer our clients advanced solutions to move their programs forward.
Our comprehensive service offerings utilized to rapidly move your treatments to the clinic include DMPK/ADME, in vitro and in vivo pharmacology, expert study design and execution. Your in vivo pharmacology studies are run in our AAALAC-accredited rodent facility with the highest regard for animal welfare. All studies are conducted with a commitment to meeting the high-quality standards you expect, and each offering is customizable to meet your unique development needs. With expert drug development insight and strategy and skilled project management, TD2 is your partner for an efficient path to the clinic.
Explore our In Vivo and In Vitro Tumor Model Systems and Bioanalytical Services
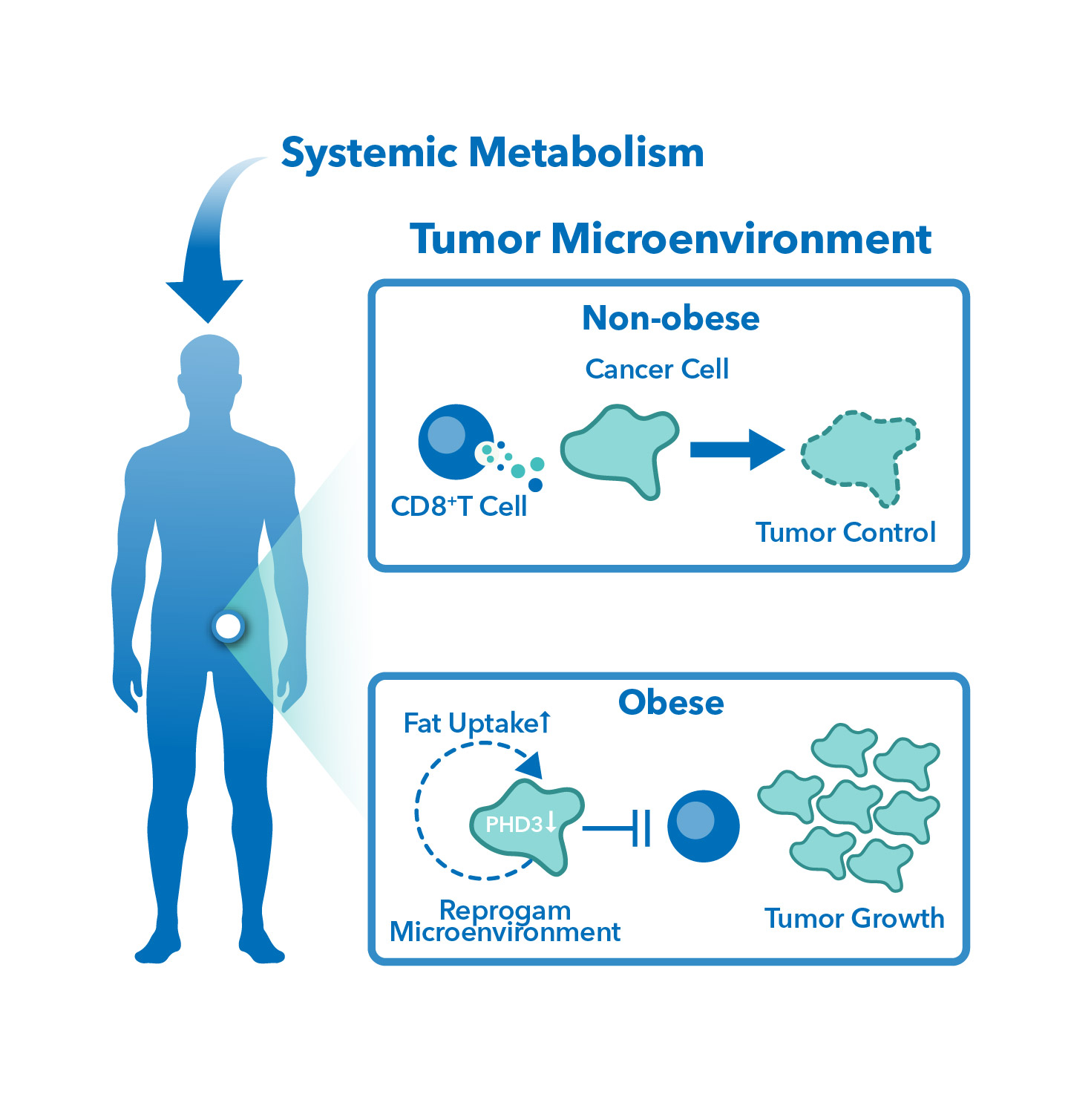
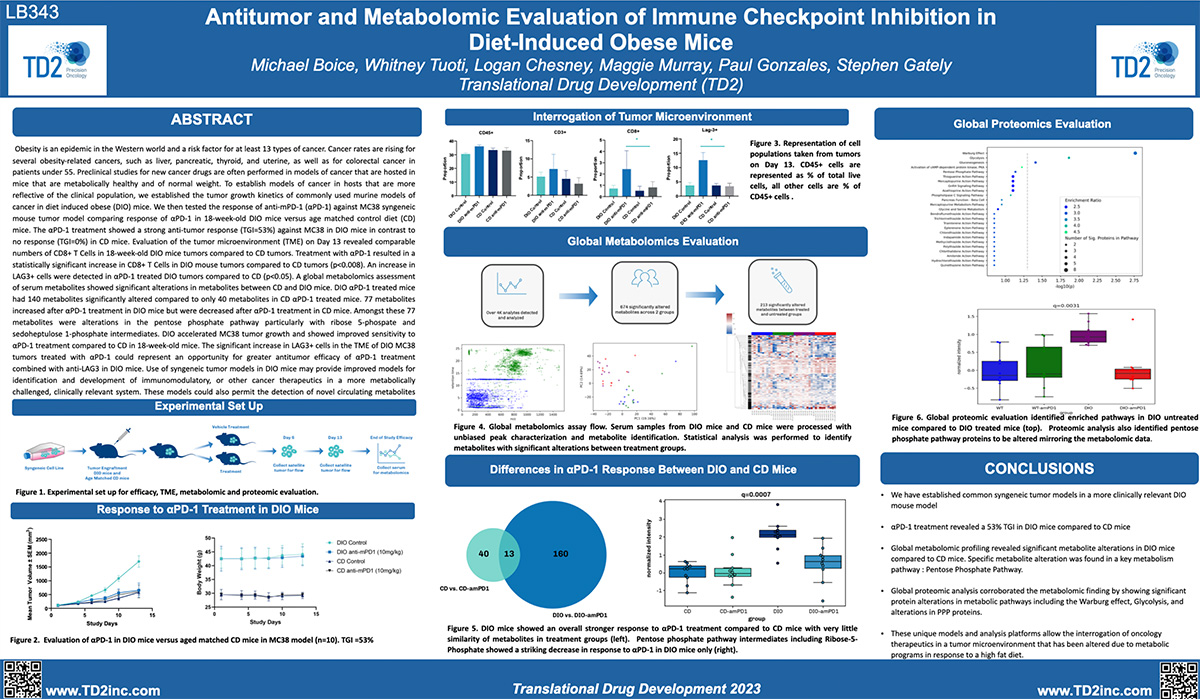
TD2 Oncology accelerates preclinical drug development with a robust suite of in vivo and in vitro tumor model systems and advanced bioanalytical services, designed to generate clinically relevant insights into drug efficacy, immune response, and tumor biology. Our diverse portfolio—including syngeneic, orthotopic, patient-derived xenograft (PDX), humanized mouse, diet-induced obesity, adoptive cell therapy, and cell line-derived xenograft (CDX) models—enables precise assessment of immuno-oncology therapies, targeted agents, and combination treatments. Integrated with flow cytometry, metabolomics and proteomics analysis, DMPK/ADME services, and non-GLP safety/tolerability assessments, our approach provides translationally relevant, data-driven support to optimize drug development strategies.
What Sets TD2 Apart in Preclinical Oncology Studies:
- Diverse & Translational Tumor Models – Clinically relevant immunocompetent, xenograft, and humanized models tailored for oncology drug discovery.
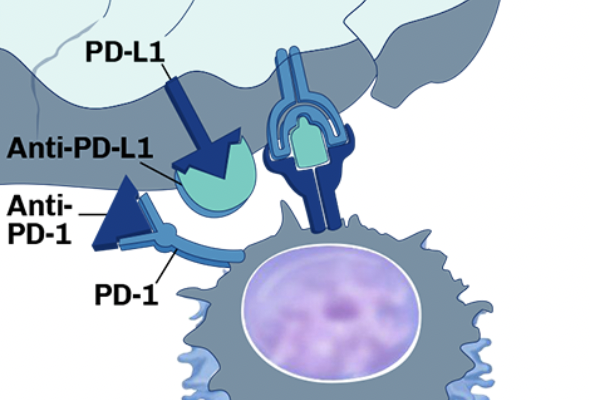
- Expertise in Immuno-Oncology – Sophisticated platforms to evaluate immune checkpoint inhibitors, cell therapies, and tumor microenvironment interactions.
- Integrated Bioanalysis Capabilities – Flow cytometry, proteomics, and metabolomics to uncover mechanistic insights and biomarker responses.
- Pharmacokinetics & Safety Profiling – DMPK/ADME and non-GLP safety/tolerability studies to optimize dosing and reduce development risks.
- Regulatory & Translational Focus – Comprehensive preclinical strategies that align with clinical and regulatory pathways to accelerate therapeutic success.
By combining state-of-the-art tumor models with highly specialized bioanalytical services, TD2 Oncology delivers actionable data to drive informed decision-making and streamline the path to clinical translation.
Therapeutic Spotlight
Additional Resources
The TD2 Oncology Ecosystem
Seamless Integration from Preclinical to Clinical Success
TD2 Oncology offers a comprehensive suite of study management services, ensuring a seamless and efficient clinical trial process from preclinical stages through regulatory approvals and beyond. Our integrated approach includes:
- Preclinical Services: Leveraging innovative technology and experienced scientists to deliver robust preclinical data which guide your clinical strategy.
- Regulatory Support: Guiding you through the complex regulatory landscape with expert advice and documentation support for an expedited path into the clinic.
- Clinical Trial Management: Providing end-to-end management of your clinical trials, from site selection and patient recruitment to data management and analysis ensuring a streamlined clinical experience.
By partnering with TD2 Oncology, you benefit from a streamlined process that minimizes delays and optimizes resource allocation, ensuring that your trial progresses smoothly at every stage. Our commitment to quality and efficiency means you can focus on your research, while we handle the operational complexities.
GET STARTED
Put your preclinical studies in the hands of a team who believes in your research as much as you do.
Are you ready to start your preclinical trial? Partner with a collaborative oncology CRO that believes in your treatment as much as you do. Take the first step today and contact our experts.