AACR 2024 Posters
Integrating PDX GBM in vivo models with patient history and whole exome sequencing: Advancing relevance and precision in preclinical studies
Abstract Number: #6908 Authors: E. Trachet, P. M. Gonzales, S. Gately
Authors: E. Trachet, P. M. Gonzales, S. Gately
Session Date and Time: Wednesday Apr 10, 2024
9:00 AM – 12:30 PM
Abrstract: Glioblastoma poses a significant challenge in oncology, necessitating improved translational models that faithfully replicate the intricate tumor microenvironment.
Patient-derived xenograft (PDX) models, are key in glioblastoma multiforme (GBM) preclinical research. PDX models (orthotopic and subcutaneous) provide a more clinically relevant representation compared to CDX xenografts. Analyzing PDX tumors in their native environment allows for a nuanced understanding of GBM biology, tumor-stroma interactions, and treatment responses. This abstract explores the rationale and advantages of utilizing PDX GBM in vivo models coupled with patient diagnosis and treatment history, empowered by tumor whole exome sequencing (WES), to enhance the relevance of preclinical studies in evaluating GBM-specific treatments. TD2 has access to over 100 GBM PDX tumors models with fully annotated patient details and treatment history. Moreover, 68 of these PDXs also have WES data, that allows most appropriate model selection to ensure alignment with the therapeutic development strategy. Below is a detailed analysis of two GBM PDX models, GBM6 and GBM46, using patient history and WES data to define criteria for success in assessing novel preclinical therapeutic agents. GBM6, was obtained prior to treatment from a 65-year-old man with glioblastoma multiforme, that contains 642 mutations, including EGFR VIII amplification. GBM46, was obtained from a 55-year-old man with prior treatment, contained 646 mutations, including RAS pathway alterations and EGFR (MET and FLT3) amplification. When grown subcutaneously the anti-tumor activity of cetuximab against GBM6 and GBM46 resulted in 41% and 48% growth inhibition, respectively. Traditional treatments have also been evaluated in the orthotopic setting, with temozolomide (TMZ) showing a nominal improvement in lifespan (11%) as expected for the unmethylated MGMT found in the GBM6 model. By contrast, GBM46 from a patient with prior treatment with TMZ, OSI-774, radiation, and BCNU, exhibited a 58% increase in overall lifespan when treated with TMZ alone. Orthotopic PDX GBM models preserve many molecular characteristics, enabling the study of intrinsic GBM mutations for developing novel therapeutic strategies. While orthotopic models better capture GBM’s dynamic nature, traditional xenograft systems remain valuable as rapid screening tools. Models with defined clinical treatment history and WES data allows for testing new agents in models that more closely resemble the clinical development path for a given new agent and aids in deciphering factors influencing therapeutic resistance. These models have the potential to reshape preclinical research paradigms, accelerating the translation of promising therapeutic agents increasing the likelihood of clinical benefit for patients with glioblastoma multiforme.
Epigenetic-based combinatorial therapy is synergistic in KRAS/LKB1 mutant non-small cell lung cancers
Abstract Number: #6491
Authors: M. Zucker, K. Devore, L. Chesney, G. Hirschfeld, A. Reyna, B. Linnane, J. Mascarenhas, M. Murray, A. Miller, P. Gonzales, S. Gately
Session Date and Time: Tuesday April 9, 2024
1:30 PM – 5:00 PM
Abrstract: KRAS and LKB1 mutations (KL) in non-small cell lung cancer (NSCLC) patients often results in poor prognosis, a high tumor mutational burden, and resistance to conventional therapy.
The metabolic changes with these mutations can create unique vulnerabilities to be targeted therapeutically. HDAC inhibition has been reported as a promising approach to overcome MEK inhibitor resistance in animal models of melanoma, pancreatic cancer, and KL mutant NSCLC. Inhibition of HDAC3 and HDAC6 have independently been shown to have therapeutic activity in KL NSCLC. We now report on the activity of two novel HDAC3/6 inhibitors in cell culture and mouse models of human KL (A549) compared to KRAS/TP53-mutant NSCLC (KP) (CALU-6) alone and individually combined with the MEK inhibitor trametinib. GB-1101 inhibits HDAC3 and HDAC6 and GB-3103 inhibits HDAC1,3,6,10. SynergyFinder multi-dose combination data analytics showed synergistic interactions for both GB-1101 or GB-3103 when combined with trametinib in KL mutant A549 cells with maximum synergy scores of δ = 9.24 and 4.21, using zero interaction potency (ZIP) for synergy scoring. GB-1101 or GB-3103 when combined with trametinib in KP mutant CALU-6 cells did not demonstrate synergistic interaction with synergy scores of δ = -1.58 and -0.53, respectively. Mice were inoculated subcutaneously with 5.0×106 A549 or CALU-6 cells and treatment with GB-1101 (75 mg/kg qd, oral), GB-3103 (5.0 mg/kg qd, subcutaneous), trametinib (1.0 mg/kg qd, oral) and each drug in combination with trametinib was initiated when tumors reached an average of 120mm3. Single agent and combination treatments were well tolerated. GB-1101 exhibited tumor growth inhibition (TGI) of 33.0% in the A549 (KL) compared to 8.5% in CALU-6 (KP). In combination with trametinib, GB-1101 showed increased activity (TGI=92.7%) compared to trametinib alone (TGI=69.5%) in KL mutant A549. In KP mutant CALU-6 tumors, combination benefit was not observed; combination of GB-1101 and trametinib yielded a TGI=93% equivalent to trametinib alone (TGI=91%). GB-3103 resulted in a TGI of 66.7% in the KL mutant A549 and TGI= 55.9% in KP mutant CALU-6. In KL mutant A549, GB-3103 in combination with trametinib, showed increased inhibition (TGI=94.3%) compared to trametinib alone (TGI=69.5%). No combination benefit was seen for GB-3103 in KP mutant CALU-6 with the combination treatment TGI of 93.1% compared to trametinib alone TGI=90.7%. These results confirm and extend the finding that for KL mutant NSCLC, HDAC3/6 inhibitors show therapeutic benefit in combination with trametinib that is not observed in KP tumors. These data reveal a promising epigenetic-based combinatorial strategy for treating lung cancers harboring concurrent KRAS/LKB1 mutations, a path for clinical translation, and the potential to improve treatments for KRAS/LKB1 mutant NSCLC patients where there are currently no robust therapeutic strategies.
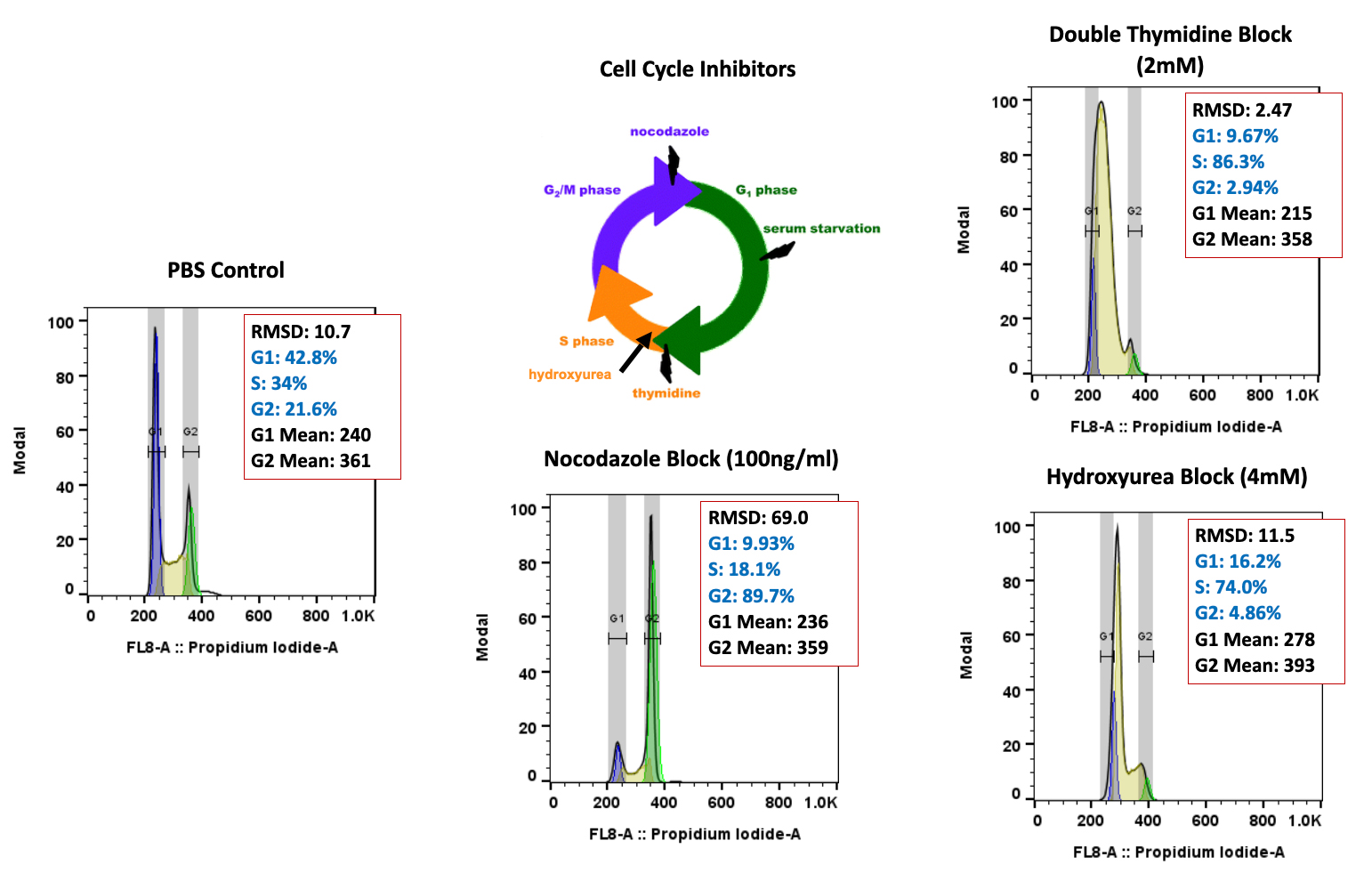
Flow cytometry evaluation of cell cycle arrest in A549 human non-small cell lung cancer cells and tumors following treatment with cell cycle inhibitors: A multifaceted approach to targeting cancer
Abstract Number: #510 Authors: E. Trachet, E. Cluff, A. Lemos de Matos, D. Diaz, C. Holmquist, P. Gonzales
Authors: E. Trachet, E. Cluff, A. Lemos de Matos, D. Diaz, C. Holmquist, P. Gonzales
Session Date and Time: Sunday April 7, 2024
1:30 PM – 5:00 PM
Abrstract: The contemporary landscape of cancer treatment has witnessed significant progress with FDA-approved targeted drugs.
The FDA’s Project Optimus has underscored the growing need for innovative pharmacodynamic endpoints to determine the optimal biological dose in clinical development. Flow cytometry, a potent analytical tool in clinical and preclinical labs, now plays a leading role in cancer research, enabling the evaluation of new drug effects on cell cycle regulation and consideration of rational drug combinations with approved drugs. At TD2, we strive to enhance the translatability of in vitro induced cell cycle arrest studies to in vivo biomarker proof of concept and efficacy. We employ a comprehensive approach, utilizing flow cytometry to assess cell cycle dynamics in cultured cells and in vivo tumor models after treatment with approved and novel drugs. This abstract focuses on the translational relevance of combined in vitro and in vivo testing of well-known drugs that disrupt the cell cycle, laying the foundation for optimized treatment strategies and potentially personalized cancer therapies. Human A549 non-small cell lung carcinoma cells were treated with either thymidine (2.0mM) or the microtubule-depolymerizing drug nocodazole (100ng/ml) for 18 hours, then examined by flow cytometry. Thymidine induced a greater than 85% S-phase arrest, while nocodazole induced a greater than 88% G2/M-phase arrest. A549 cells (5×106 cells/mouse) were subcutaneously implanted in SCID mice, followed by treatment with an FDA-approved cell cycle inhibitor, Palbociclib, or cisplatin chemotherapy. Palbociclib, a CDK4/6 inhibitor, and low-dose cisplatin treatment resulted in an increased accumulation of cells in the G0/G1 phase, greater than 80% for each. Both treatments exhibited promising anti-cancer activity, with tumor growth inhibition (TGI) of 69% and 87%, respectively. Our findings highlight flow cytometry’s multifaceted utility in assessing cell cycle arrest, providing a comprehensive view of agent effects on cancer cells in culture and within the complex in vivo tumor microenvironment. Determining the impact of any new drug on specific cell cycle checkpoints could allow for a more strategic plan for combination therapy. Integrating flow cytometry could enable early biomarker monitoring of tumor cell cycle changes, offering insights into the therapeutic potential of new agents alone or in combination. This multidimensional evaluation contributes to understanding cancer biology and has the potential to guide the development of novel therapeutic strategies, optimize treatment regimens to identify optimal biological doses, and improve patient outcomes in the era of precision medicine.