Resources
Regulatory, Clinical, Preclinical, PK/ADME, Flow
Comprehensive Oncology Ecosystem
Regulatory, Preclinical, Videos
Advancing Antibody-Drug Conjugate Therapies: Key Preclinical and Regulatory Strategies for Clinical Success
Preclinical
Model and Bioanalytical Services Catalog
Preclinical
Validated Tumor Cell Lines
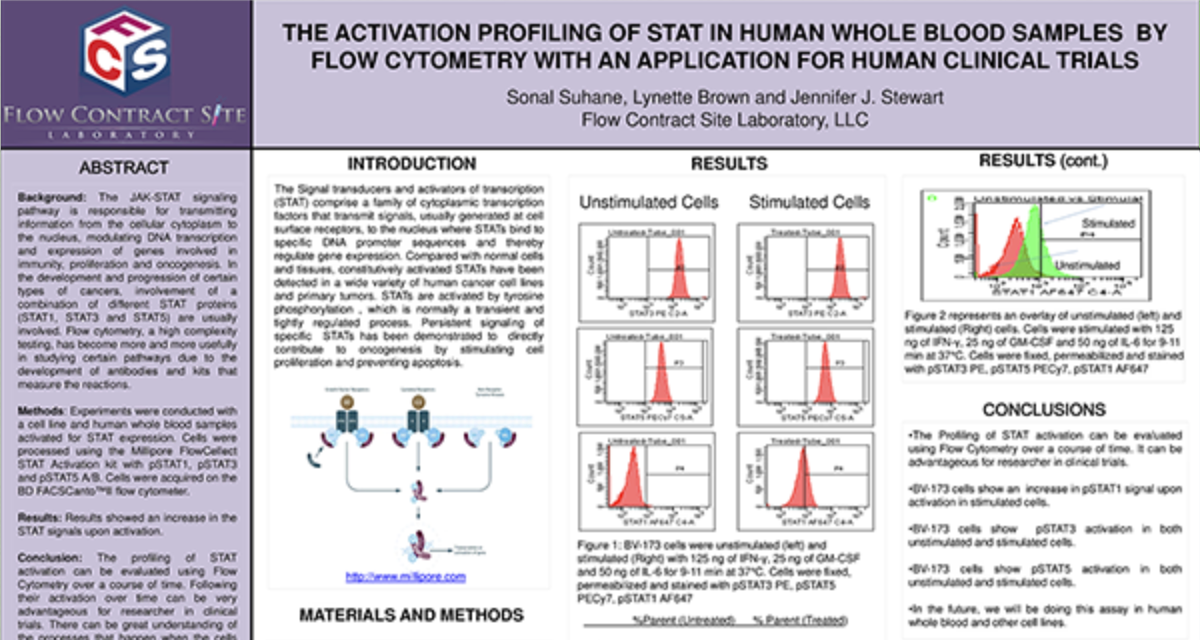
Clinical
Navigating Project Optimus with TD2
Preclinical, Posters and Publications
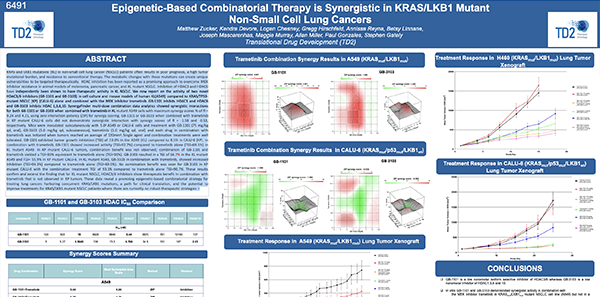
Epigenetic-Based Combinatorial Therapy is Synergistic in KRAS/LKB1 Mutant Non-Small Cell Lung Cancers
Preclinical, Posters and Publications
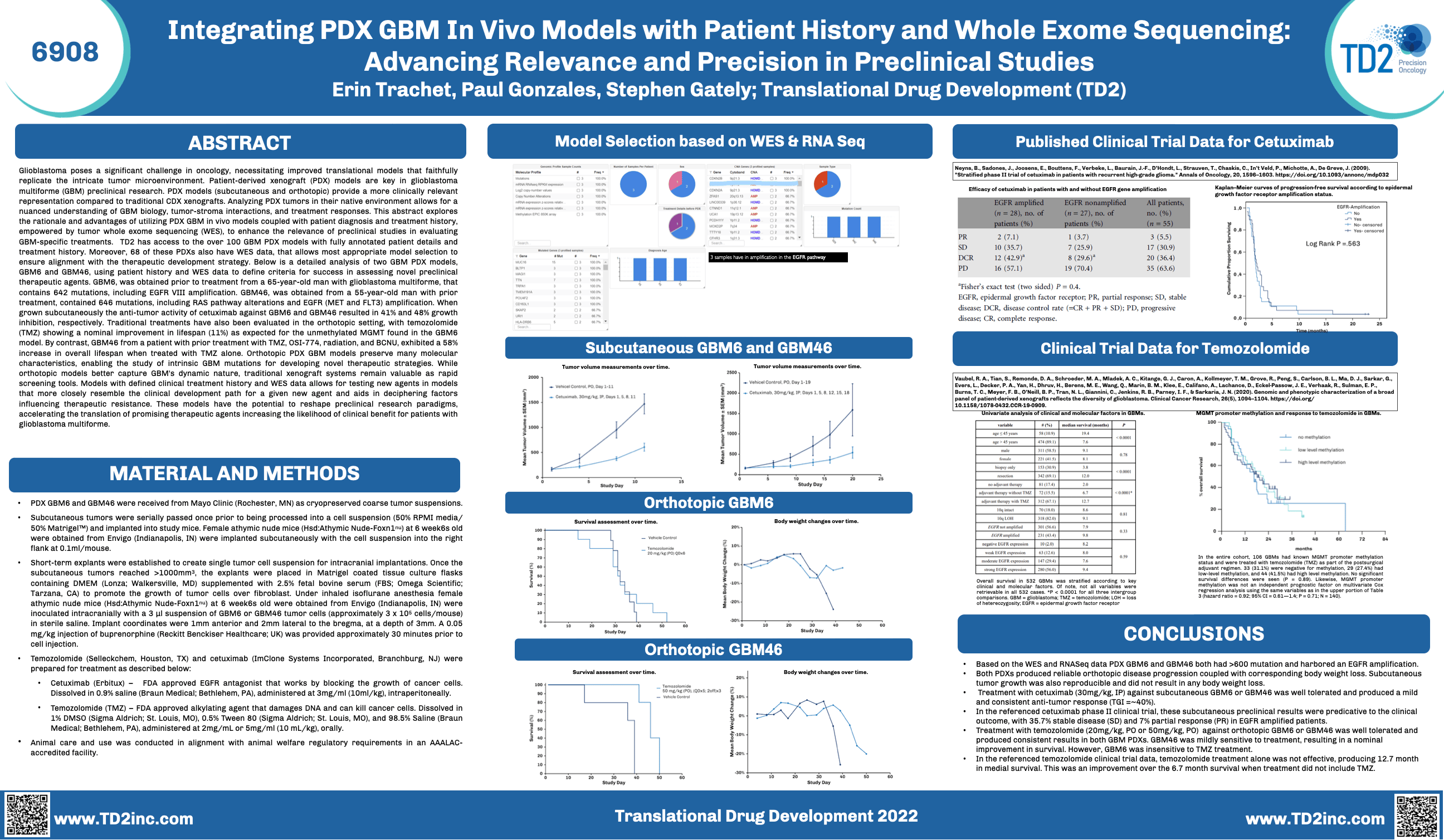
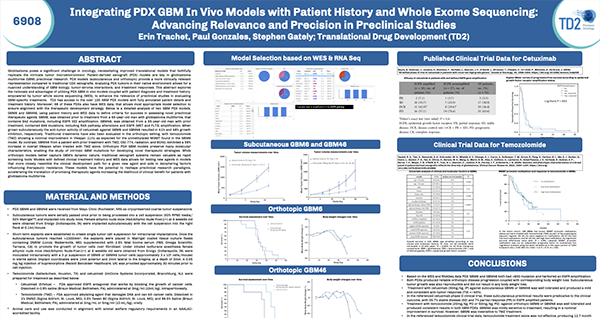
Integrating PDX GBM In Vivo Models with Patient History and Whole Exome Sequencing: Advancing Relevance and Precision in Preclinical Studies
Preclinical, Posters and Publications
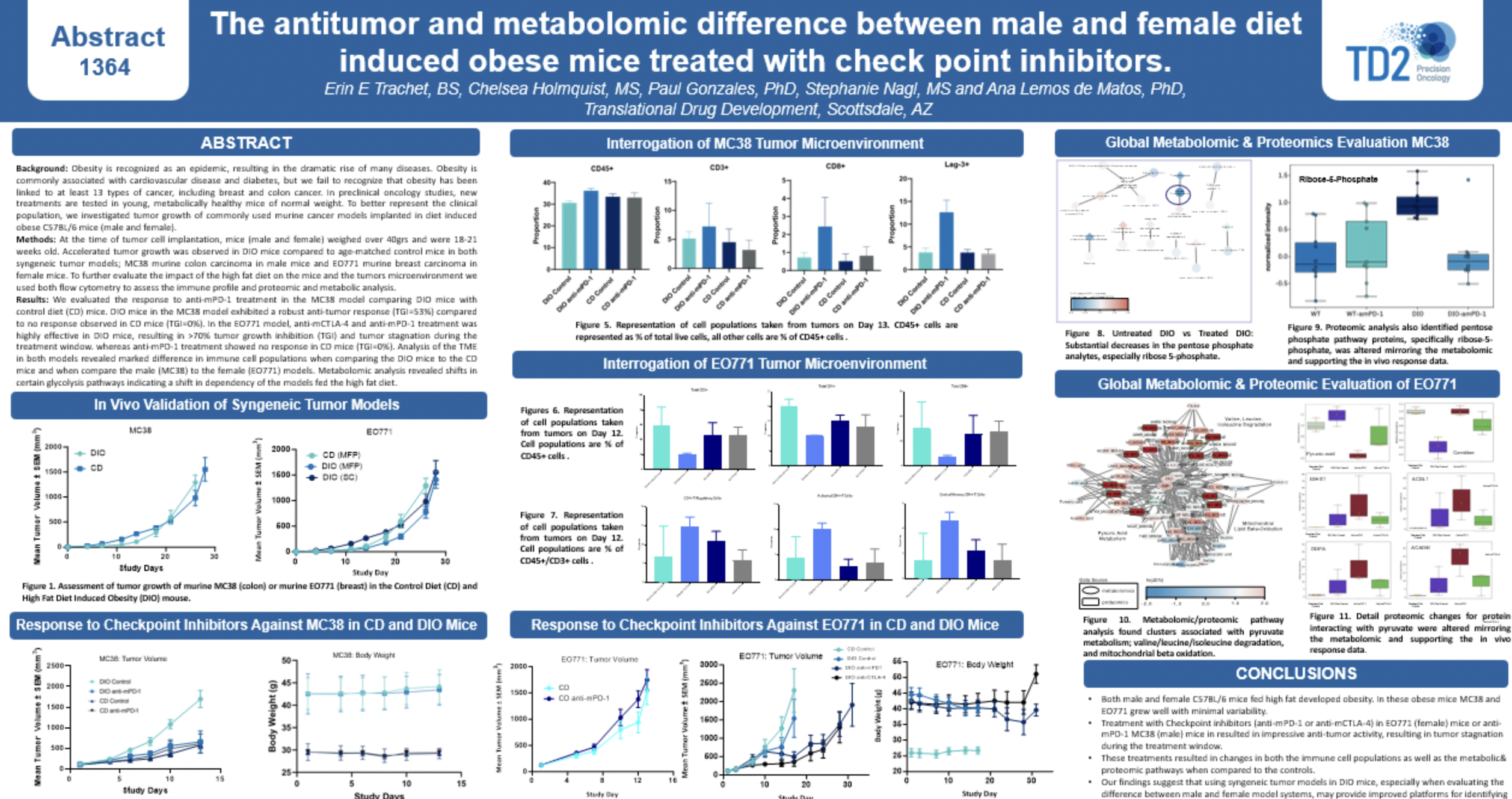
E-Book: Using the Diet Induced Obese Mouse Model for Modern Cancer Research
Preclinical, Posters and Publications
A Dual-specific Inhibitor of Rock/Aurk, RR-1752, for Primary Myelofibrosis
Preclinical, Posters and Publications
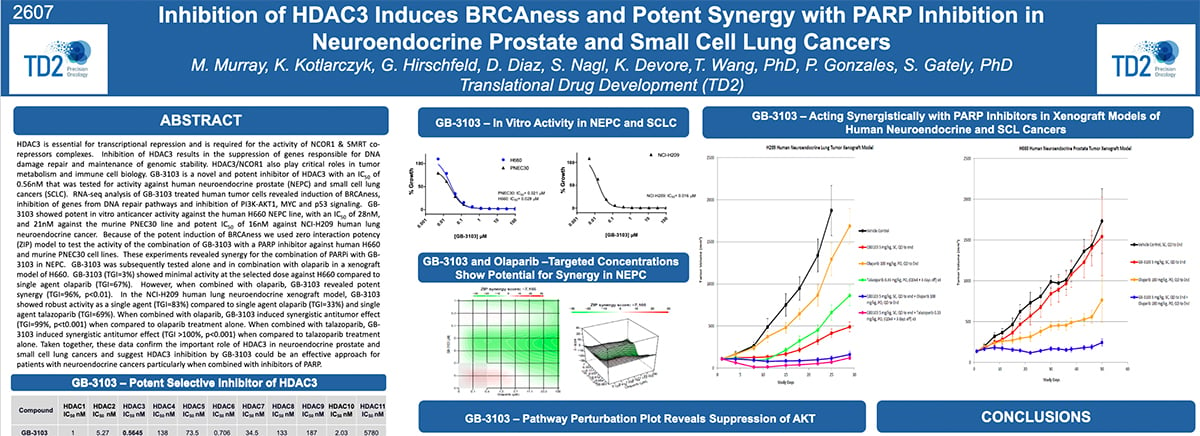
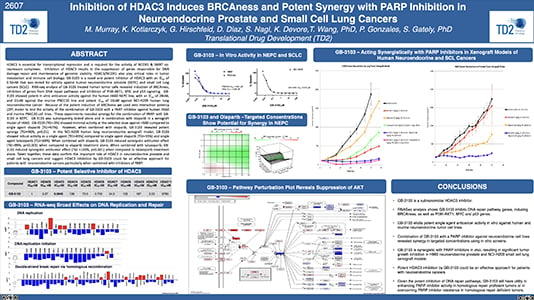
Inhibition of HDAC3 Induces BRCAness and Potent Synergy with PARP Inhibition in Neuroendocrine Prostate and Small Cell Lung Cancers
Preclinical, Posters and Publications
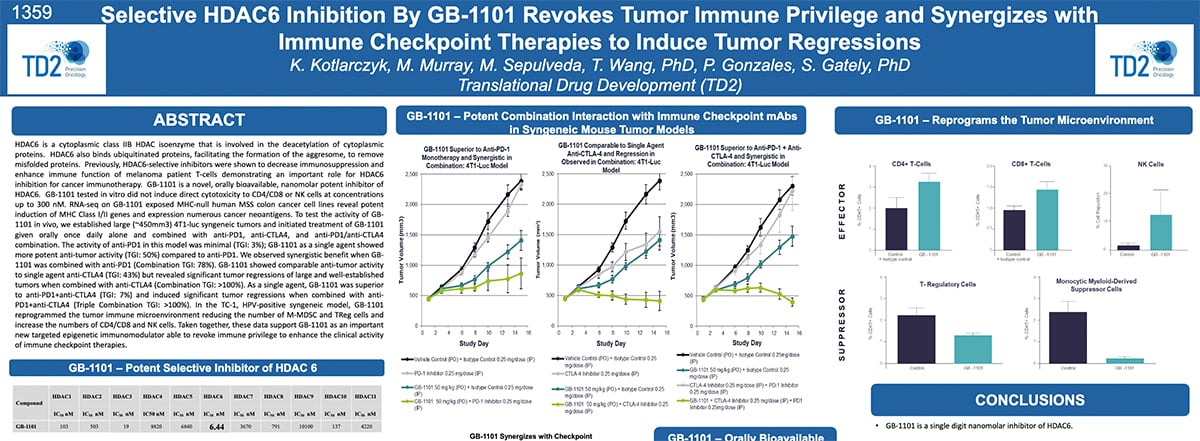
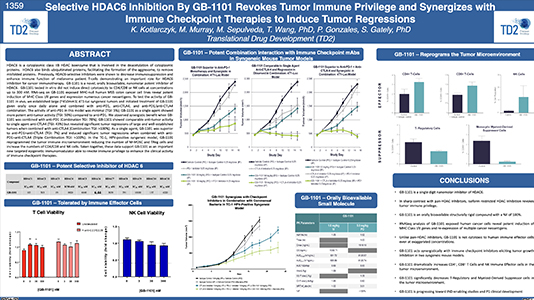
Selective HDAC6 Inhibition By GB-1101 Revokes Tumor Immune Privilege and Synergizes with Immune Checkpoint Therapies to Induce Tumor Regressions
Preclinical, Posters and Publications
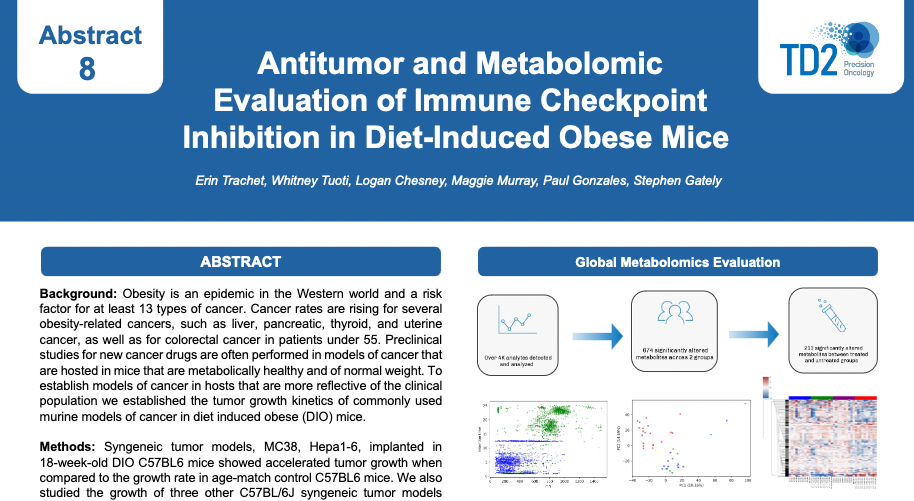
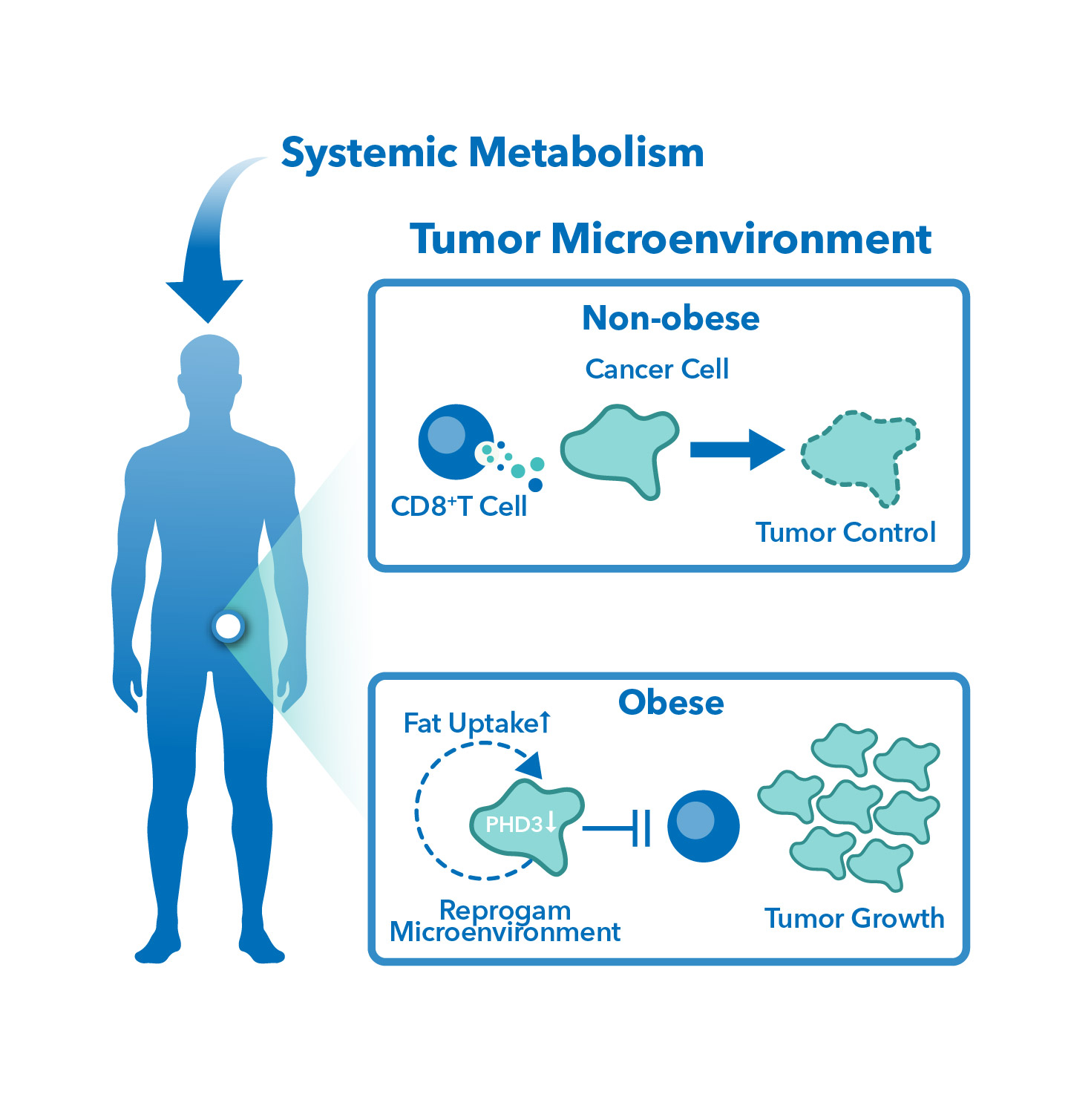
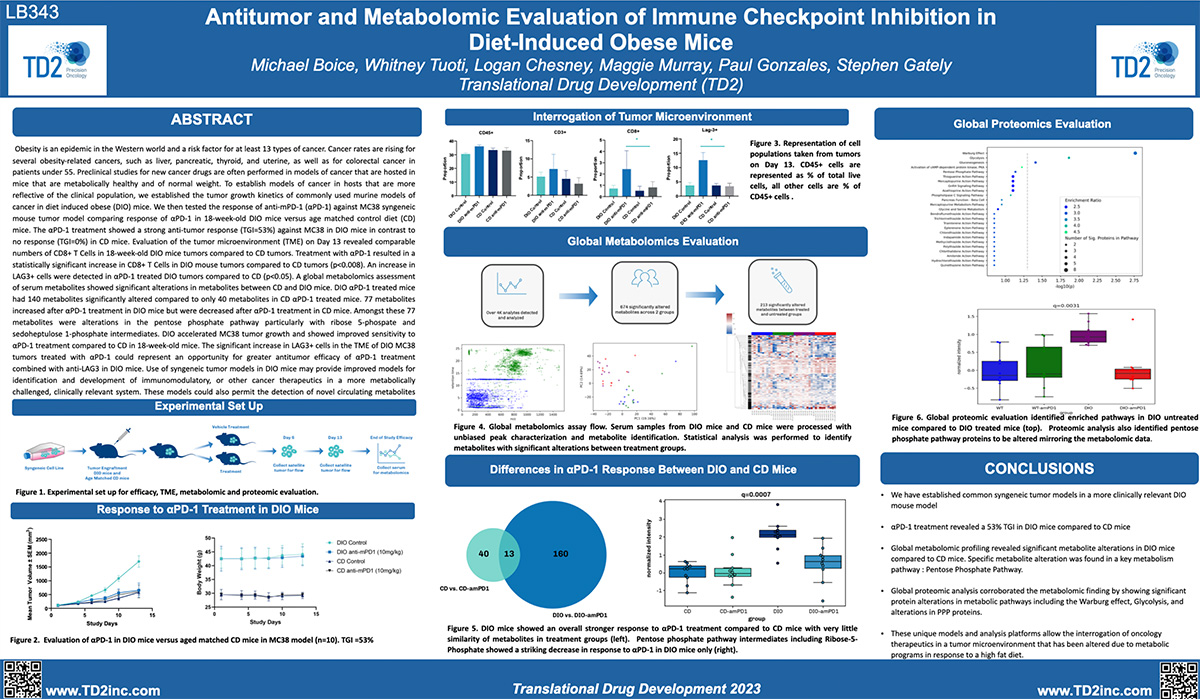
SITC 2023 Poster: Antitumor and metabolomic evaluation of immune checkpoint inhibition in diet-induced obese mice
Preclinical, Posters and Publications
Metabolomics Sample Data Analysis Report
Preclinical, Posters and Publications
Antitumor and metabolomic evaluation of immune checkpoint inhibition in diet-induced obese mice
Clinical, Preclinical, Posters and Publications
Leading the way in defining a new Immunotherapy Response Score (IRS) to predict checkpoint inhibitor benefit
Clinical, White Papers
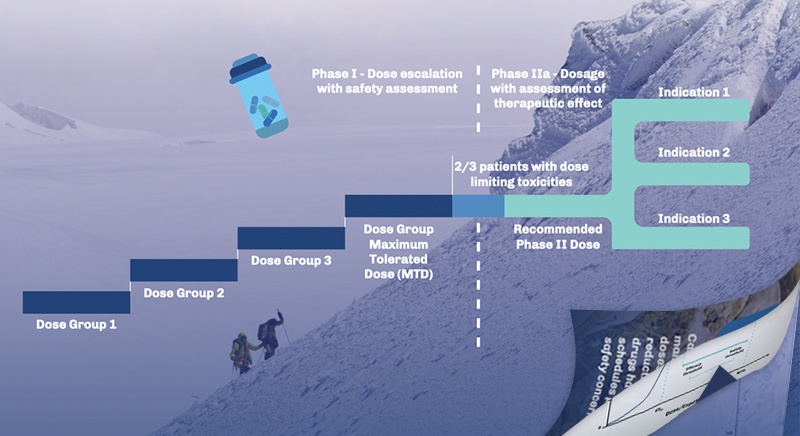
Early Phase Oncology Trial Designs Phase I Strategies Tailored for Success
Videos
TD2 Immuno-Oncology
Videos
Executive Interview with TD2
Videos
TD2 Clinical Capabilities
Videos
Cell Therapy at TD2
Videos
CAR-T Summit Tech Slam
Videos
Clinical Trial Highlight
Videos
Glioblastoma Capabilities
Preclinical, Webinars
The Dynamic Trial Design
Preclinical, Webinars
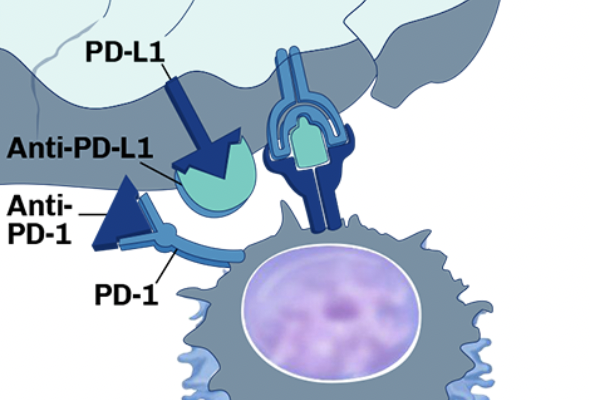
Checkpoint inhibitors: the gut microbiome’s role in anti-tumor response
Preclinical, Posters and Publications
Inhibition of HDAC3 Induces BRCAness and Potent Synergy with PARP Inhibition in Neuroendocrine Prostate and Small Cell Lung Cancers
Preclinical, Posters and Publications
Selective HDAC6 Inhibition By GB-1101 Revokes Tumor Immune Privilege and Synergizes with Immune Checkpoint Therapies to…
Preclinical, White Papers
Exploring the Effects of Microbiome in Precision Oncology Therapeutic Development
Clinical, White Papers
Improving Cancer Trial Recruitment With Advanced Analytics and Prospective Data
Clinical, Posters and Publications
Data Insights: Identifying Early Signs of Safety and Efficacy in Oncology Clinical Trials
Preclinical, White Papers
Many Drugs Don’t Move Past Phase I. Will Yours?
Preclinical, White Papers
CAR T-Cell Therapy: Revolutionizing Cancer Treatment
Preclinical, White Papers