Flow Cytometry Services
Integrated flow cytometry solutions supporting global drug development programs
We offer comprehensive flow cytometry services to support drug development across research and development, preclinical research, and clinical trials. Flow cytometry enables high resolution analysis, allowing precise immunophenotyping, biomarker evaluation, and functional assessment of complex immune and disease relevant cell populations. Our flow cytometry experts partner closely with sponsors to design and execute flow cytometry assays that generate reproducible, high-quality data to inform translational strategy and clinical decision making.
Our flow cytometry capabilities support a broad range of therapeutic areas, including but not limited to oncology, autoimmune disease, allergy, and inflammatory disorders, as well as advanced modalities such as cell and gene therapy and biologics. From preclinical flow cytometry studies focused on mechanism of action and pharmacodynamics to clinical flow cytometry assays supporting biomarker development and clinical endpoints, we deliver customized, solutions aligned with regulatory expectations and program timelines.
Life Cycle of a Potential Therapeutic


Request more information about our Flow Cytometry Services
Contact our experts to help advance your drug development with TD2's trusted Flow Cytometry Services.
Capabilities and Assays Include:
![]() Tissue Dissociation/Single-Cell Suspension
Tissue Dissociation/Single-Cell Suspension
- Spleen, Tumors, Lymph nodes
![]() Primary Cell Isolation
Primary Cell Isolation
- Pan T cells isolation kit, mouse
- CD4+ T cell isolation kit, mouse
- isolation of untouched mouse cells from single-cell suspensions from spleen
![]() Immune Population Phenotypingx
Immune Population Phenotypingx
- Panel design 20+ colors
- Whole blood, bone marrow, spleen, and tumor
- Viability dye inclusion
- Surface staining
- Intracellular staining, including:
- Cytoplasmic proteins
- Phospo-proteins (specific buffer set)
- Nuclear transcription factors (specific buffer set)
- Cytokines (specific buffer set)
- T-lymphocyte, B-lymphocyte and Natural Killer cells (TBNK)
- T Regulatory Cells
- T Memory, Naive and Effector Cells
- T Cell Activation
- B Memory and Plasma cells
- Humanized mice panels
- Custom panels
- CAP TBNK Panel
![]() Cancer Cells/Tumor Specific Marker Expressionx
Cancer Cells/Tumor Specific Marker Expressionx
- Viability dye inclusion
- Cell lines and single-cell suspension tumors
- Surface staining
![]() Fluorescent Proteins Expressionx
Fluorescent Proteins Expressionx
- Viability dye inclusion
- Cell lines and transfected primary cells
- Surface staining
![]() Cell Cycle Analysisx
Cell Cycle Analysisx
- Propidium iodide
- Cell lines
- Solid tumors (under development)
![]() Intracellular Protein Analysisx
Intracellular Protein Analysisx
- Intracellular Cytokines
- Phosphorylated Proteins
- NK Cell Function
- Basophil Activation Test (BAT)
Additional Resources
GET STARTED
Work with a team who believes in your research as much as you do.
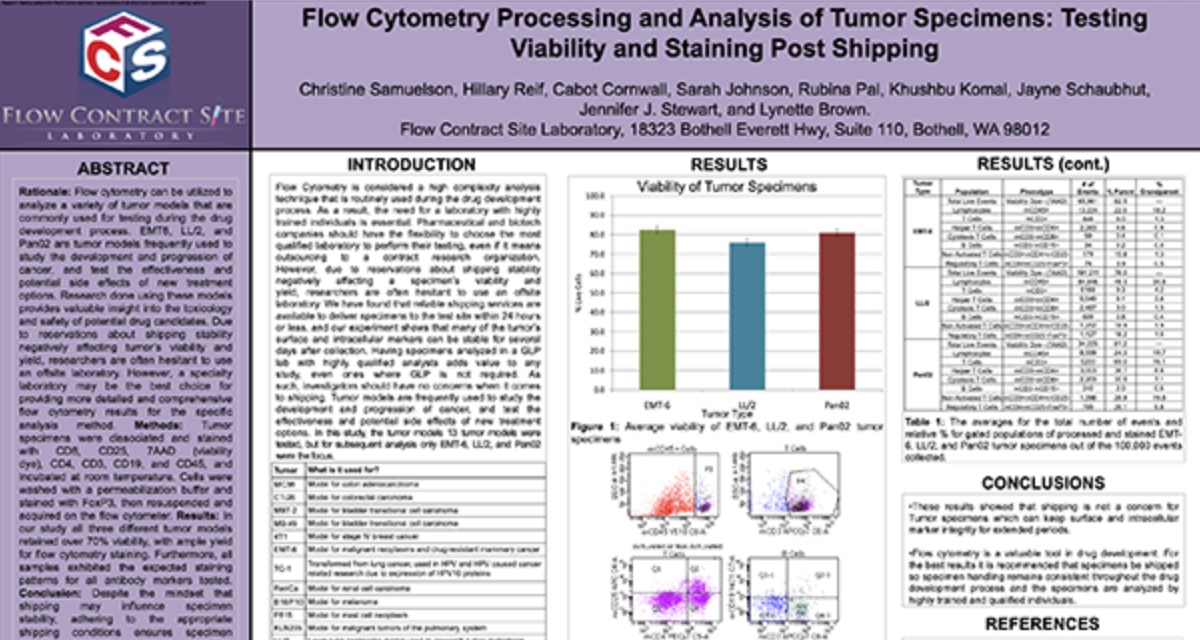
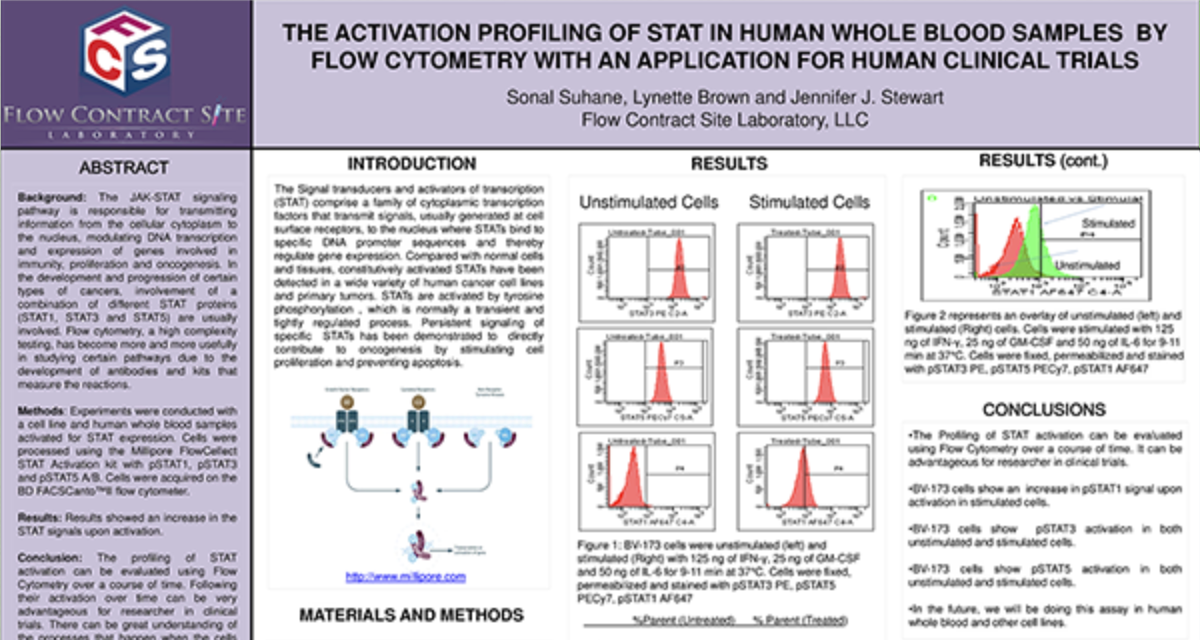
Are you ready to start your Flow Cytometry studies? Partner with a collaborative oncology CRO that believes in your treatment as much as you do. Take the first step today and contact our experts.
![]() Receptor Occupancy
Receptor Occupancy
Binding of fluorophore labeled “drug” in relation to decreasing dose of unlabeled “drug”

![]() Receptor Occupancy
Receptor Occupancy
![]() PBMC Services
PBMC Services
Evaluate responses of PBMCs by profiling the function/phenotype of subpopulations

![]() Immune Population Phenotyping
Immune Population Phenotyping
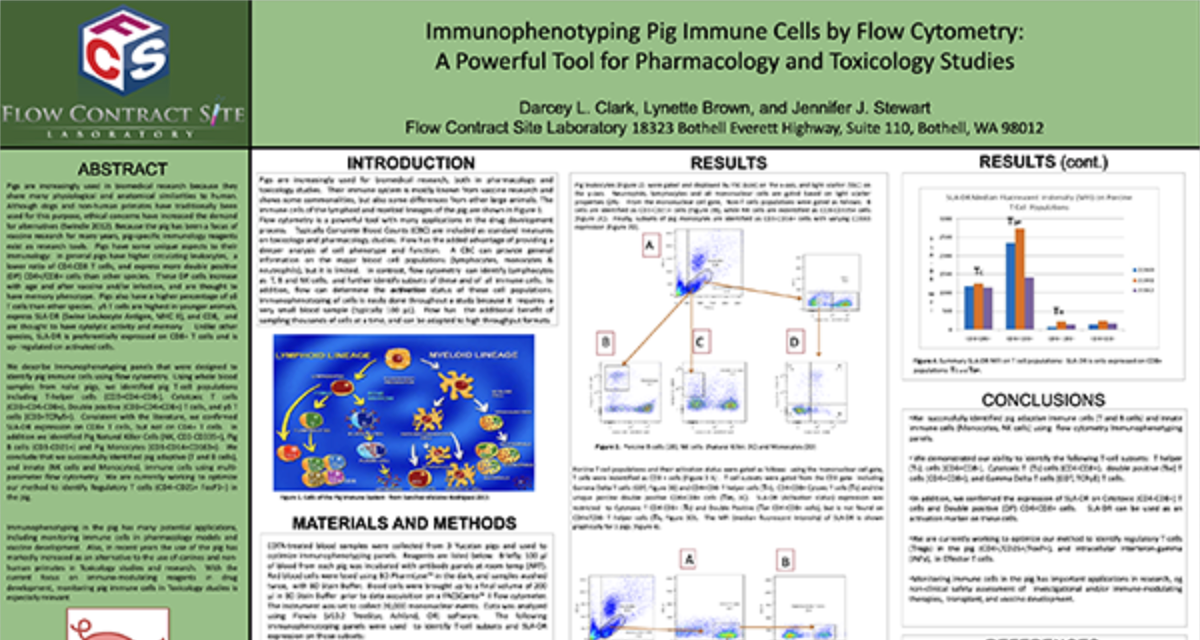
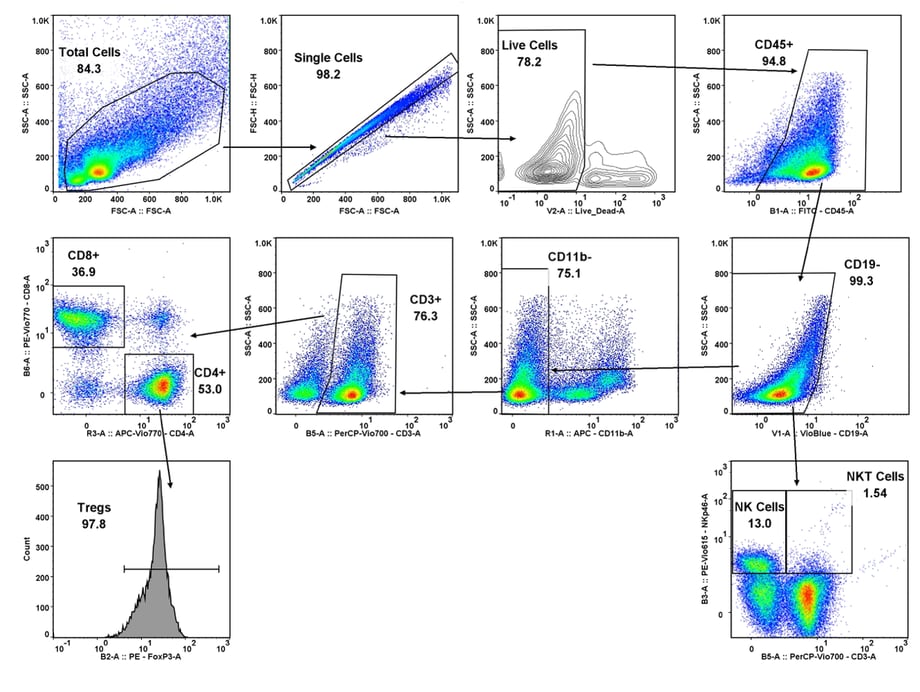
Immune cell population analysis of subcutaneous CT26 murine colon carcinoma tumors
CT26 SC tumors: Gating strategy T cells and NK cells.
Gating involved identifying the total cell population, and then gate out the doublets, gate out the dead cells, move on to our first marker CD45 which gates out any non-immune cells. The bottom right cytogram shows the different NK cell populations. The far left middle cytogram depicts the two T cell population CD4+ and CD8+. Values are % of total CD45+ cells.
![]() Cancer Cells/Tumor Specific Marker Expression
Cancer Cells/Tumor Specific Marker Expression
Cancer cells/tumors specific markers expression
- Viability dye inclusion
- Cell lines and single-cell suspension tumors
- Surface staining

![]() Fluorescent Proteins Expression
Fluorescent Proteins Expression
Fluorescent proteins expression
- Viability dye inclusion
- Cell lines and transfected primary cells
- Surface staining

![]() Cell Cycle Analysis
Cell Cycle Analysis
Cell cycle analysis A549 cells
- Propidium iodide
- Cell lines

![]() Intracellular Protein Analysis
Intracellular Protein Analysis
- Intracellular Cytokines
- Phosphorylated Proteins
- T-Regs
- Phosphoroteins
- Cytokines

![]() Cellular Functional Assays
Cellular Functional Assays
- NK Cell Function
- Basophil Activation Test (BAT)