Clinical Trial Services for Small Molecules
DEEP EXPERTISE IN SMALL MOLECULE DRUG DEVELOPMENT
Unlock your small molecule's full potential with our specialized clinical trial services, designed to accelerate development from first-in-human through regulatory approval.
We have extensive experience navigating the unique complexities of small molecule oncology therapeutics, delivering data-driven strategies and seamless execution across all phases of clinical development.
Proven Track Record
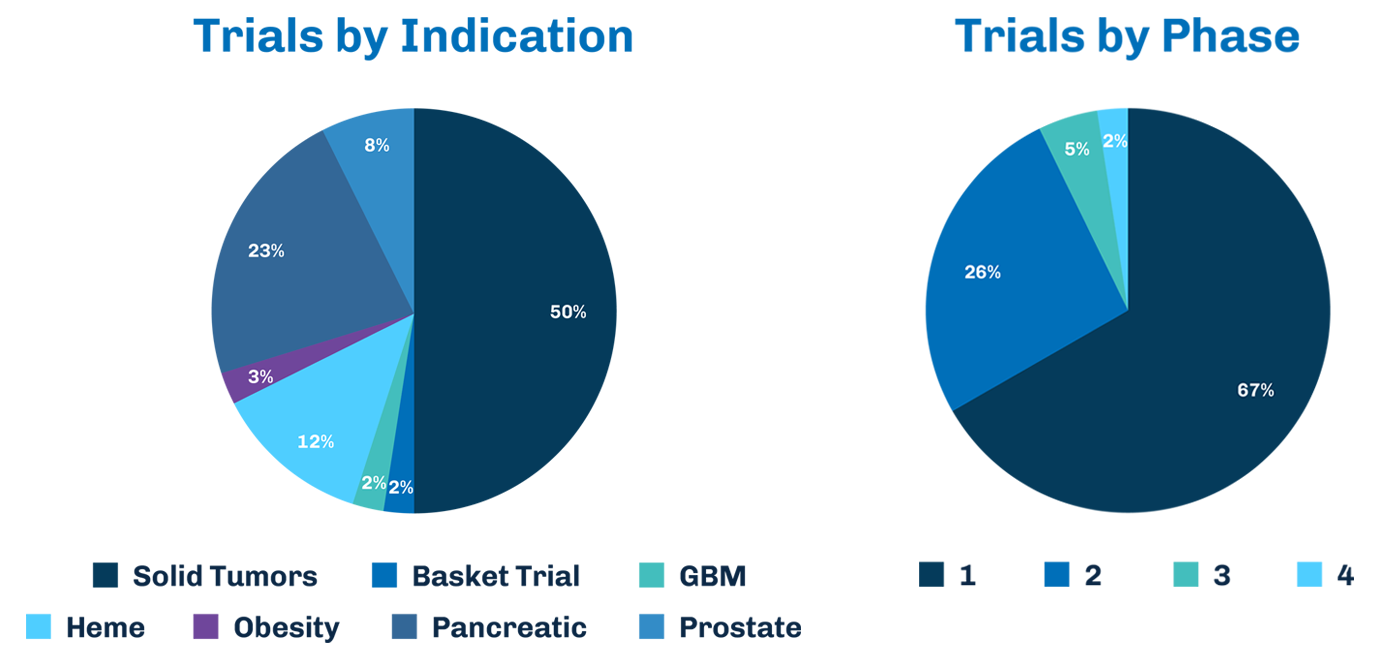
With a proven history of successfully executed small molecule clinical trials and numerous regulatory successes, TD2 is your trusted partner for accelerating oncology drug development.
Your Small Molecule, Optimized for Success
Contact us today to discuss how our specialized clinical trial services can streamline your small molecule's path to market.
Clinical Trial Services for Small Molecules
- Strategic Protocol Design: Expertly crafted protocols that proactively manage drug resistance through adaptive trial designs and combination therapy strategies.
- Clinical Operations and Site Management: Selection and management of clinical sites skilled in effectively managing patient enrollment and retention, addressing narrow therapeutic windows and optimizing dosing schedules.
- Comprehensive PK/PD Analysis: Specialized pharmacokinetic and pharmacodynamic studies to ensure drug bioavailability, solubility, stability, and metabolism optimization.
- Regulatory Navigation: Experienced guidance to navigate complex regulatory landscapes, including addressing bioequivalence, quality control, and formulation stability requirements.
- Specialized Medical Monitoring: Oncology-specific clinical monitoring to swiftly identify and mitigate off-target toxicities and adverse effects, optimizing patient safety and trial integrity.
- Precision Biomarker Integration: Development and integration of robust biomarker strategies to enhance patient stratification and accurately predict therapeutic responses.
- Data Analytics and Biostatistics: Advanced analytics tailored to handle small molecule-specific complexities, effectively managing data integrity, drug-drug interactions, and efficacy assessments.
- Robust Pharmacovigilance: Proactive pharmacovigilance systems designed to quickly identify and address drug interactions, toxicity profiles, and adverse reactions unique to small molecule oncology therapies.
Features and Benefits of Working with TD2:
- End-to-End Expertise: Comprehensive support throughout the clinical lifecycle, tailored specifically for small molecule oncology development.
- Accelerated Timelines: Streamlined operational strategies significantly reduce time-to-market.
- Scientific and Clinical Precision: Oncology-focused expertise ensures optimal trial design and execution.
- Collaborative Approach: Transparent, aligned communication to integrate seamlessly with your internal teams.
- Proven Regulatory Success: Extensive experience successfully navigating regulatory submissions and approvals, significantly mitigating risk.
Additional Resources
![]()
Comprehensive
Clinical Trial Services Include:
GET STARTED
Put your clinical trial in the hands of a team who believes in your research as much as you do.
Are you ready to start your preclinical trial? Partner with a collaborative oncology CRO that believes in your treatment as much as you do. Take the first step today and contact our experts.



