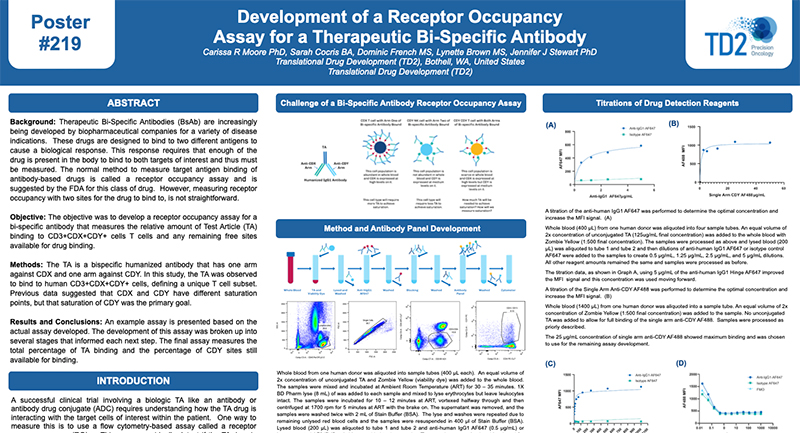
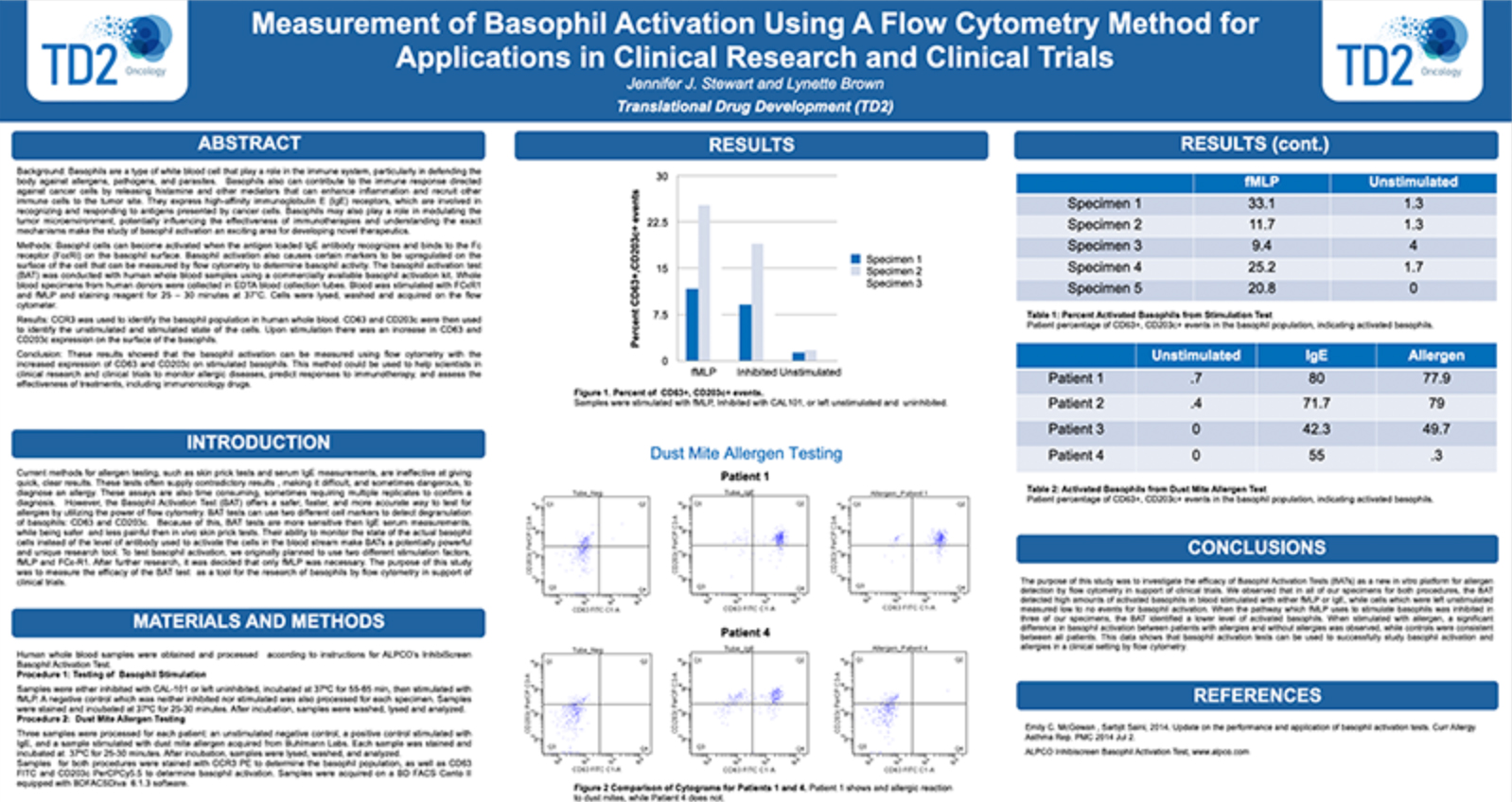
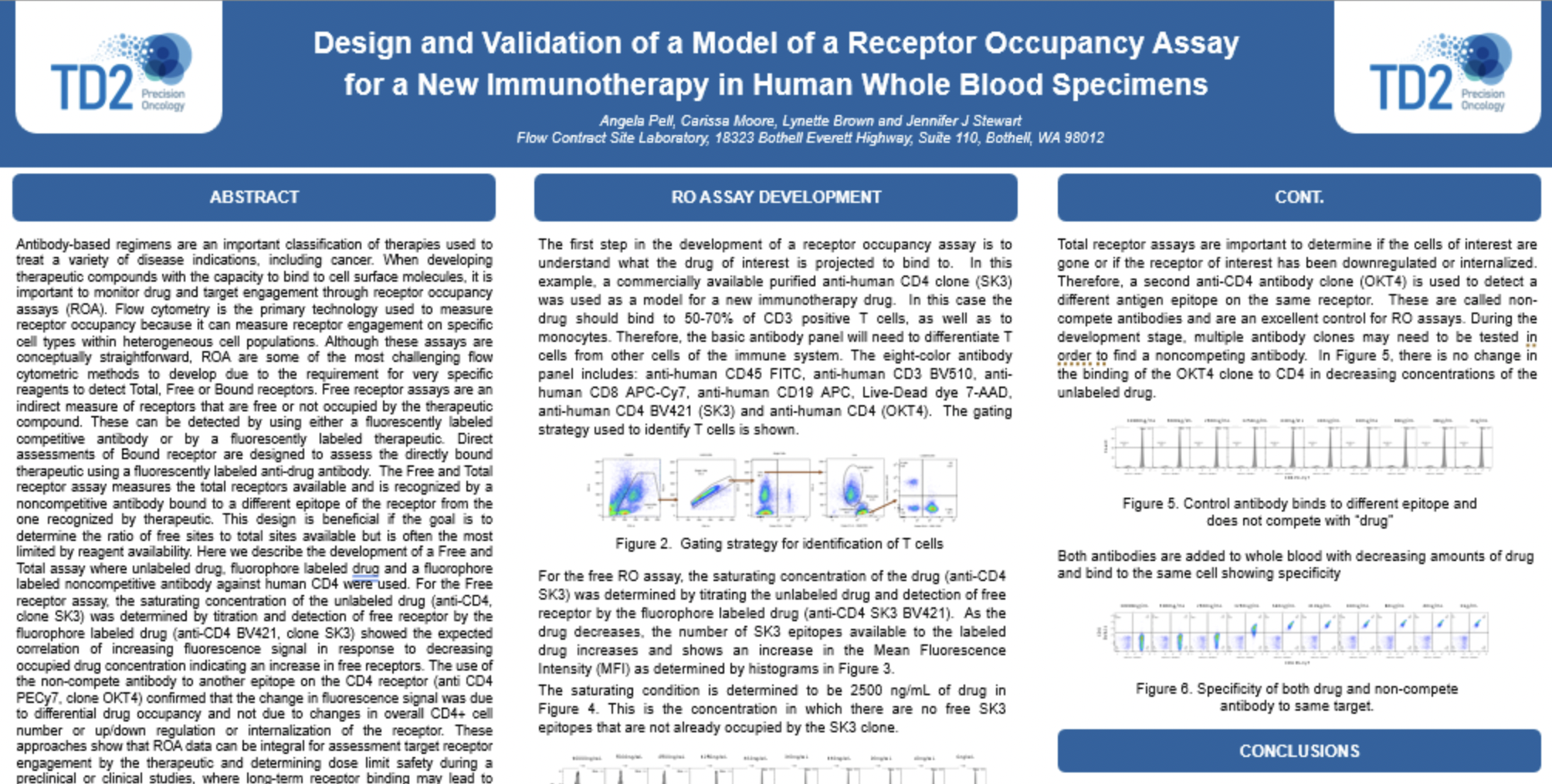
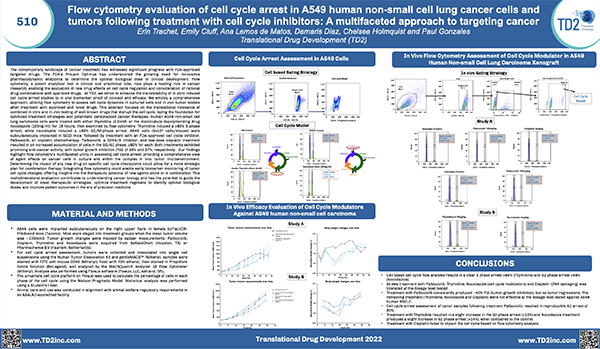
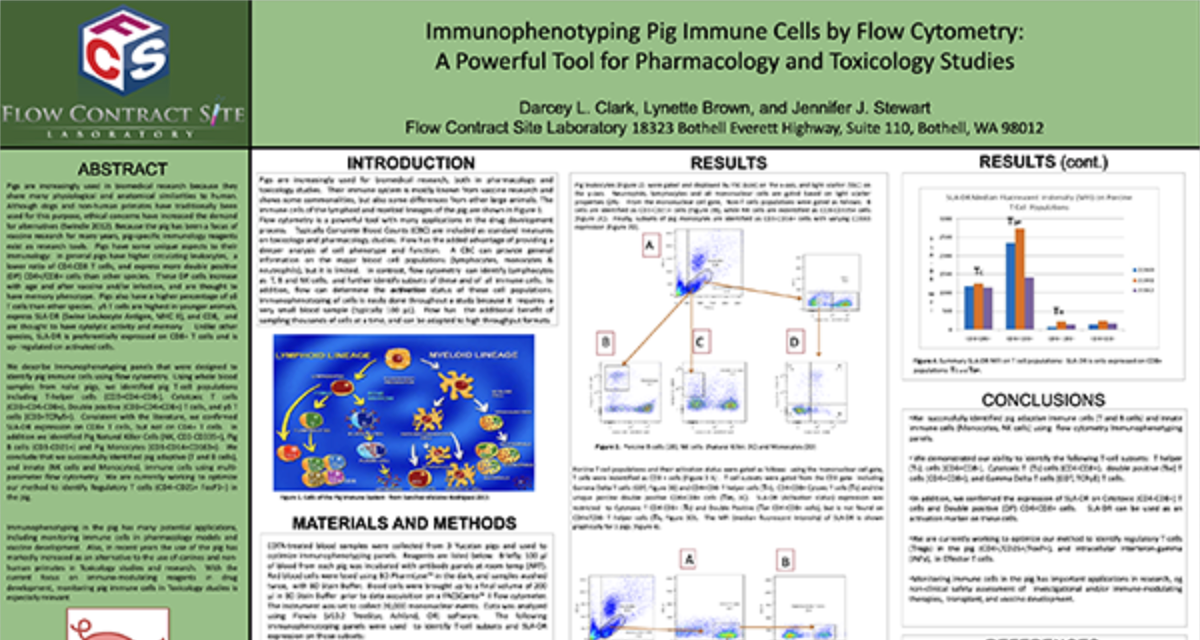
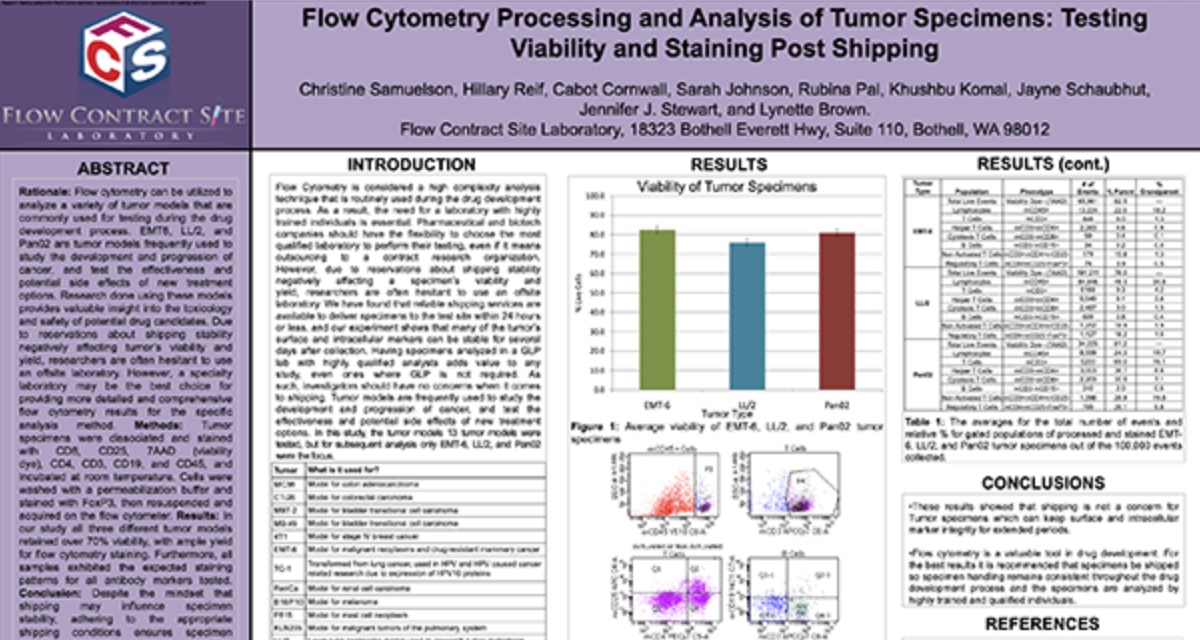
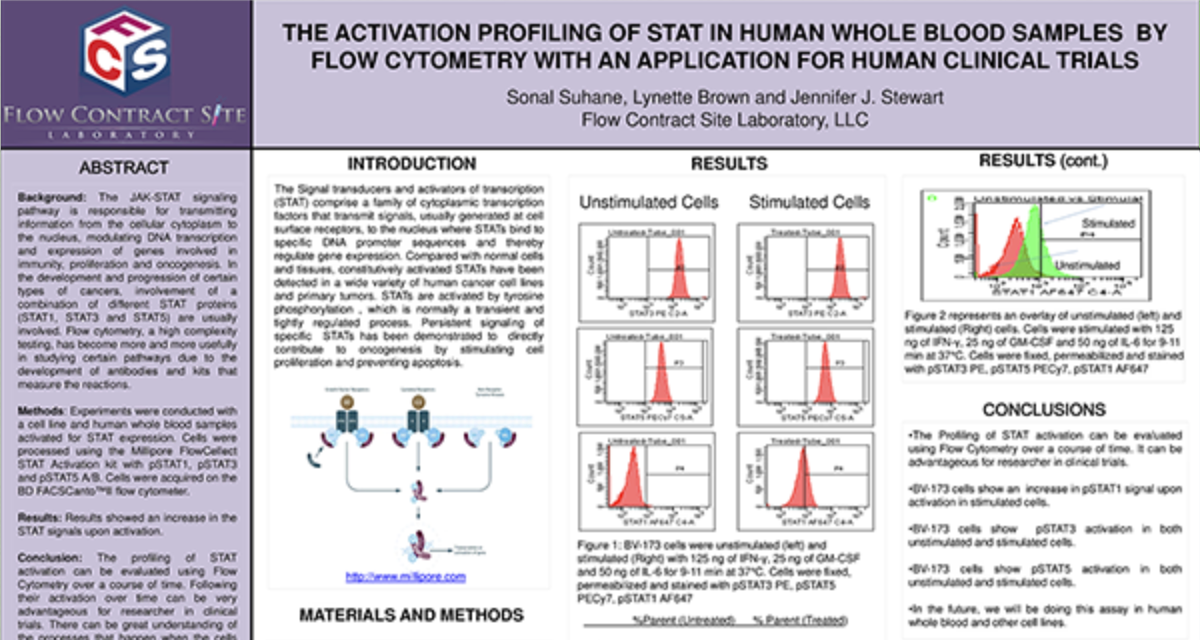
Peripheral Blood Mononuclear Cells (PBMC)
The separation of Peripheral Blood Mononuclear Cells (PBMC) may be required for certain applications including flow cytometry. At FCSL we can provide support for PBMC separation as required for your specific assay. Please inquire to start the scientific discussion.
Most flow cytometry assays can be performed on whole blood or on samples that have been drawn and undergone further processing. For some applications it may be ideal to prepare processed whole blood in order to store cells for longer periods than fresh blood or to perform batch testing for each subject over several time points. In these instances, peripheral blood mononuclear cells (PBMCs) -are prepared and frozen. PBMCs consist of lymphocytes (T cells, B cells& NK cells), monocytes and dendritic cells. In humans, lymphocytes make up the majority of the PBMC population, followed by monocytes and only a small percentage of dendritic cells PBMCs are the critical component of the immune system that fights infections and diseases. Therefore, key immune responses can be evaluated using PBMCs by profiling - the function and/or phenotype of the different PBMC subpopulations.
PBMCs must be separated from whole blood. At TD2, we -use density gradient separation for most of our PBMC preparations.

Figure 1. PBMC isolation by Density gradient centrifugation
Request more information about Peripheral Blood Mononuclear Cells
Contact our experts to help advance your drug development with TD2's trusted Flow Cytometry Services.
As shown in Figure 1, each cell population exhibits a unique migration pattern through the medium and each layer contains specific blood components. The cells are isolated by extracting the respective layer. Isolated PBMCs are washed and then evaluated for cell viability and cell count - using the Guava PCA (Personal Cell Analysis) as shown in Figure 2 and 3.

Figure 2. Guava PCA flow Cytometer

Figure 3. Cell viability and viable cell count by Guava PCA
Viable PBMCs are cryopreserved in freezing media. Our laboratory has both liquid nitrogen and -80° freezers for short term storage until the specific flow cytometry assay can be performed or shipped to other laboratories for other downstream applications.
At TD2 we have extensive experience in PBMC isolation and cryopreservation. We can help your next PBMC projects with ensuring high cell viability and recovery, timely, standardized and quality results. Please inquire to start the scientific discussion.
Additional Resources
GET STARTED
Work with a team who believes in your research as much as you do.
Are you ready to start your Flow Cytometry studies? Partner with a collaborative oncology CRO that believes in your treatment as much as you do. Take the first step today and contact our experts.
![]() Receptor Occupancy
Receptor Occupancy
Binding of fluorophore labeled “drug” in relation to decreasing dose of unlabeled “drug”

![]() Receptor Occupancy
Receptor Occupancy
![]() PBMC Services
PBMC Services
Evaluate responses of PBMCs by profiling the function/phenotype of subpopulations

![]() Immune Population Phenotyping
Immune Population Phenotyping
Immune cell population analysis of subcutaneous CT26 murine colon carcinoma tumors
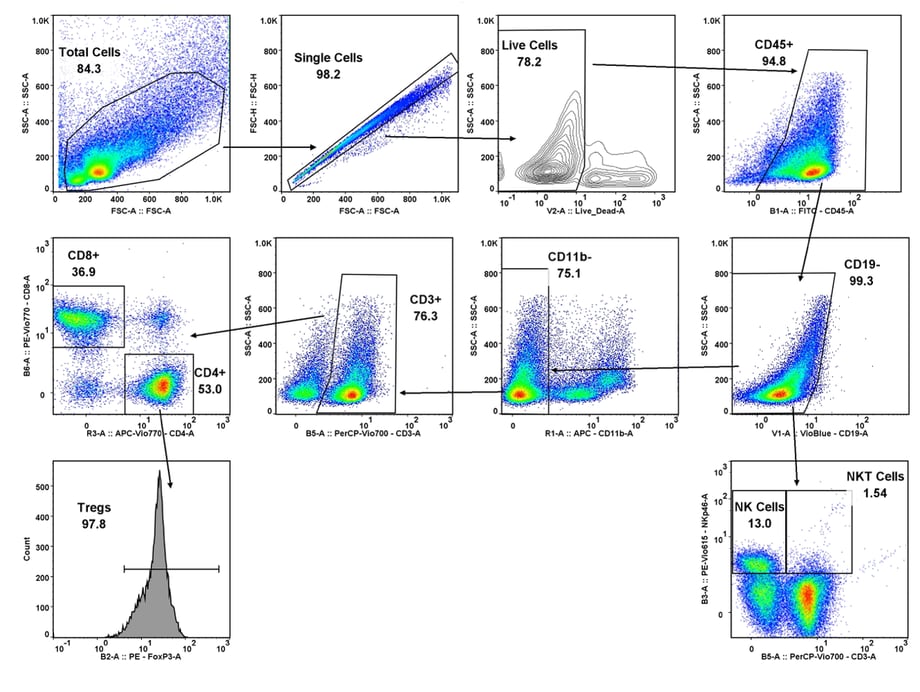
CT26 SC tumors: Gating strategy T cells and NK cells.
Gating involved identifying the total cell population, and then gate out the doublets, gate out the dead cells, move on to our first marker CD45 which gates out any non-immune cells. The bottom right cytogram shows the different NK cell populations. The far left middle cytogram depicts the two T cell population CD4+ and CD8+. Values are % of total CD45+ cells.
![]() Cancer Cells/Tumor Specific Marker Expression
Cancer Cells/Tumor Specific Marker Expression
Cancer cells/tumors specific markers expression
- Viability dye inclusion
- Cell lines and single-cell suspension tumors
- Surface staining

![]() Fluorescent Proteins Expression
Fluorescent Proteins Expression
Fluorescent proteins expression
- Viability dye inclusion
- Cell lines and transfected primary cells
- Surface staining

![]() Cell Cycle Analysis
Cell Cycle Analysis
Cell cycle analysis A549 cells
- Propidium iodide
- Cell lines

![]() Intracellular Protein Analysis
Intracellular Protein Analysis
- Intracellular Cytokines
- Phosphorylated Proteins
- T-Regs
- Phosphoroteins
- Cytokines

![]() Cellular Functional Assays
Cellular Functional Assays
- NK Cell Function
- Basophil Activation Test (BAT)