Explore TD2’s Clinical Trial Enabling Program
Fast-Track Your Trial Start-Up
TD2’s Clinical Trial Enabling Program, part of our unique Oncology Ecosystem, is designed to streamline processes and optimize resource allocation from IND submission to the enrollment of the first patient. By addressing critical operational activities before IND submission, we significantly reduce monthly trial oversight costs and minimize the time between trial operations and subject enrollment.
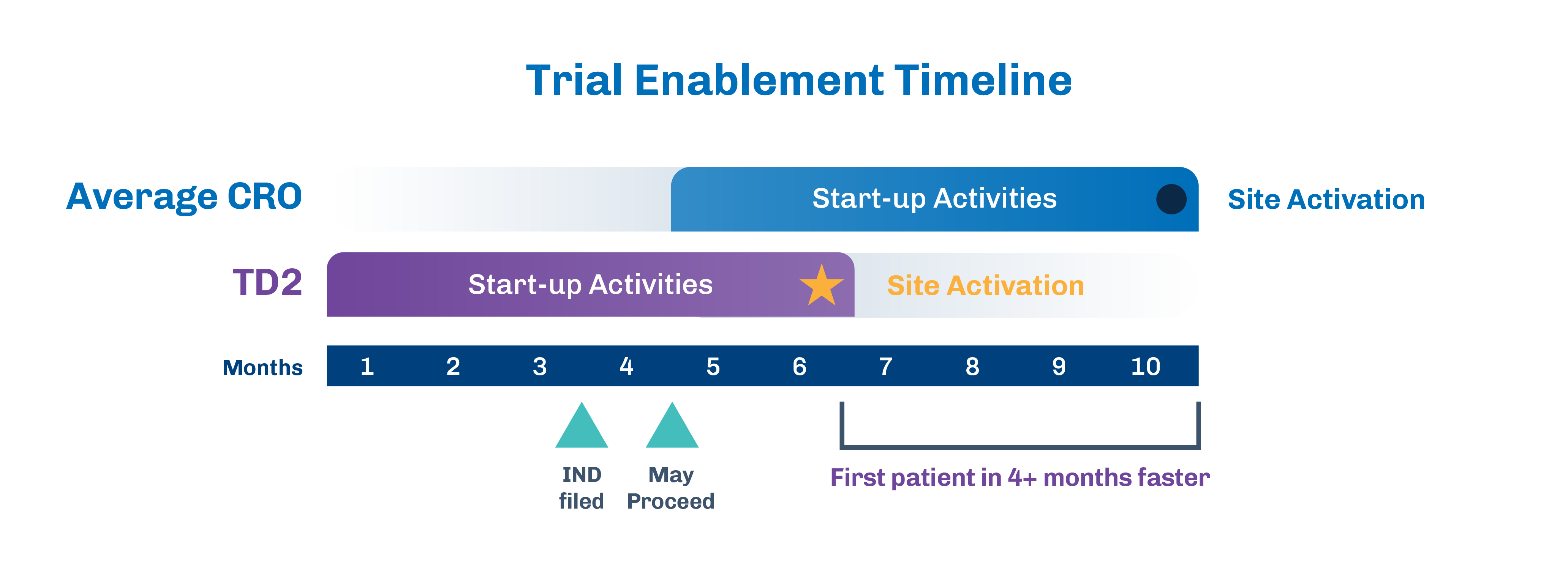
We initiate our Clinical Trial Enabling Services 4 months prior to IND filing, ensuring vendor contracts and manuals are in place upon receipt of your “May Proceed” Letter. Industry-standard timelines from May Proceed Letter to site activation typically span 6 months or more. However, with our expert guidance, target timelines are reduced to 6-8 weeks for site activation and 8-10 weeks to First Patient In.
Request more information about our Clinical Trial Enabling Program
Learn more about how our Clinical Trial Enabling Program can help you achieve your clinical goals.
Features and Benefits:
- Clinical Strategy and Protocol Writing: Initiate the development of comprehensive clinical strategies and protocols well in advance to align with regulatory requirements and optimize trial design.
- Regulatory Document Preparation: Complete Investigator Brochures (IB), Informed Consent Forms (ICF), Laboratory Manuals and Pharmacy Manuals early to prevent delays.
- Site-selection and Start-up: Engage with investigators and pre-select the best sites to enhance enrollment rates.
- Laboratory and Logistics Vendor Identification: Efficiently identify and contract necessary vendors through our large and trusted network, including drug depots and laboratories, ahead of time.
The TD2 Oncology Ecosystem
Seamless Integration from Preclinical to Clinical Success
TD2 offers a comprehensive suite of study management services, ensuring a seamless and efficient clinical trial process from preclinical stages through regulatory approvals and beyond. Our integrated approach includes:
- Preclinical Services: Leveraging innovative technology and experienced scientists to deliver robust preclinical data which guide your clinical strategy.
- Regulatory Support: Guiding you through the complex regulatory landscape with expert advice and documentation support for an expedited path into the clinic.
- Clinical Study Management: Providing end-to-end management of your clinical trials, from site selection and patient recruitment to data management and analysis ensuring a streamlined clinical experience.
By partnering with TD2, you benefit from a streamlined process that minimizes delays and optimizes resource allocation, ensuring that your trial progresses smoothly at every stage. Our commitment to quality and efficiency means you can focus on your research, while we handle the operational complexities.
Additional Resources
GET STARTED
It's never too soon to start planning for the success of your oncology trials.
Whether you're in the early stages of preclinical research or preparing for IND submission, TD2 is here to help accelerate your path to clinical success.
![]() Protein Binding Analysis
Protein Binding Analysis
Protein binding assays assesses the degree to which drugs attaches to proteins within the plasma/tissue. A drug’s efficacy may be affected by the degree to which it binds. TD2 has validated this assay using the Rapid Equilibrium Device and analysis done by LC-MS.
- Plasma
- Microsomal
- Tumor/Tissue

Figure 3. The process of protein binding assessment with rapid equilibrium dialysis.

Figure 4. Protein binding analysis by equilibrium dialysis of plasma across mutiple different speacies (mouse, rat, dog, monkely, human) following exposure to multiple compounds.
![]() Metabolism
Metabolism
In vitro drug metabolism studies, are a screening mechanism to characterize drug metabolites, elucidate their pathways, to help with further in vivo testing.
- Hepatic Fractions (S9/Microsomes)
- Hepatocyte
- CYP Phenotyping
- CYP Inhibition

Figure 5. Observes relationship between inhibitor (Ketoconazole) and the substrate (Midazolam).

Figure 6. Inhibition Profile for CYP3A4
![]() Permeability
Permeability
Screening tools for the evaluation of a drug’s ability to cross various biological membrane systems in a microplate format.
- PAMPA
- Caco-2

Figure 7. Example of Caco-2 Assay results. Assessment of the permeability of either Metoprolol (highly Permeable) or Quinidine (PgP substrate). A cell monolayer grown on a filtered-well is used to evaluate test compound movement between the insert (apical compartment -A) and the plate well (basal compartment -B). Passive transport through the layer is determine by A-B flux, whereas active transporter will introduce a B-A distribution of compounds between the two compartments.




