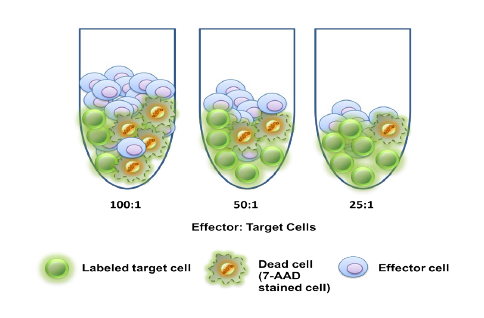
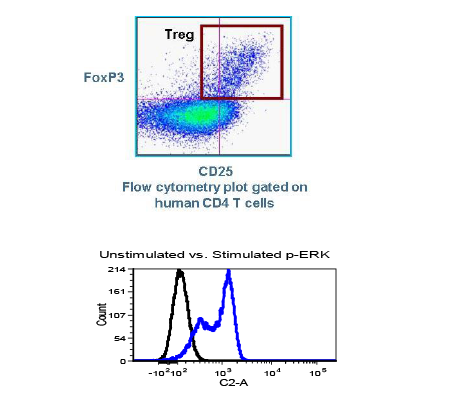
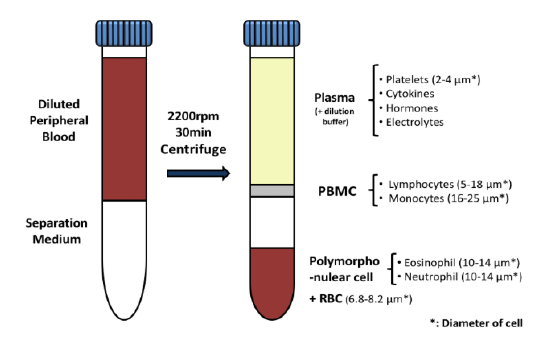
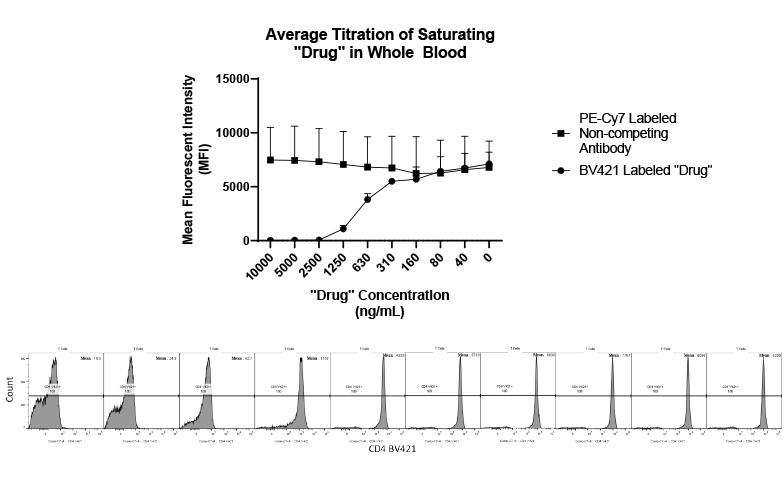
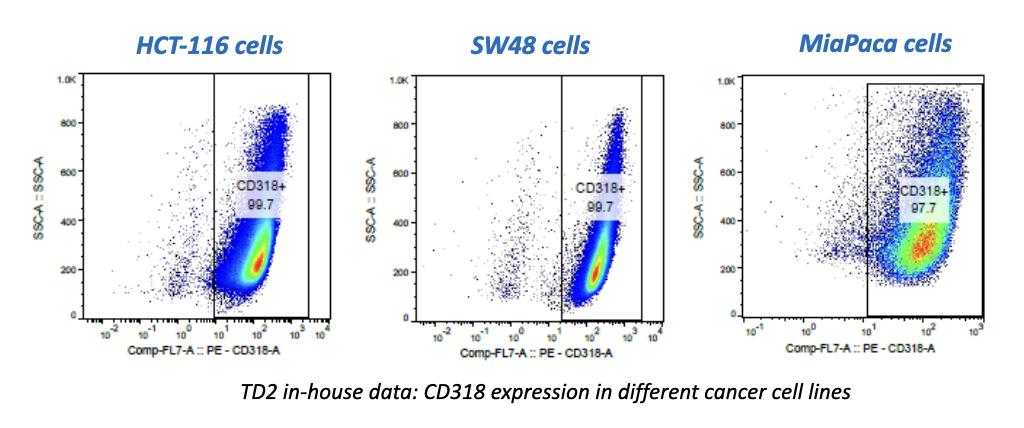
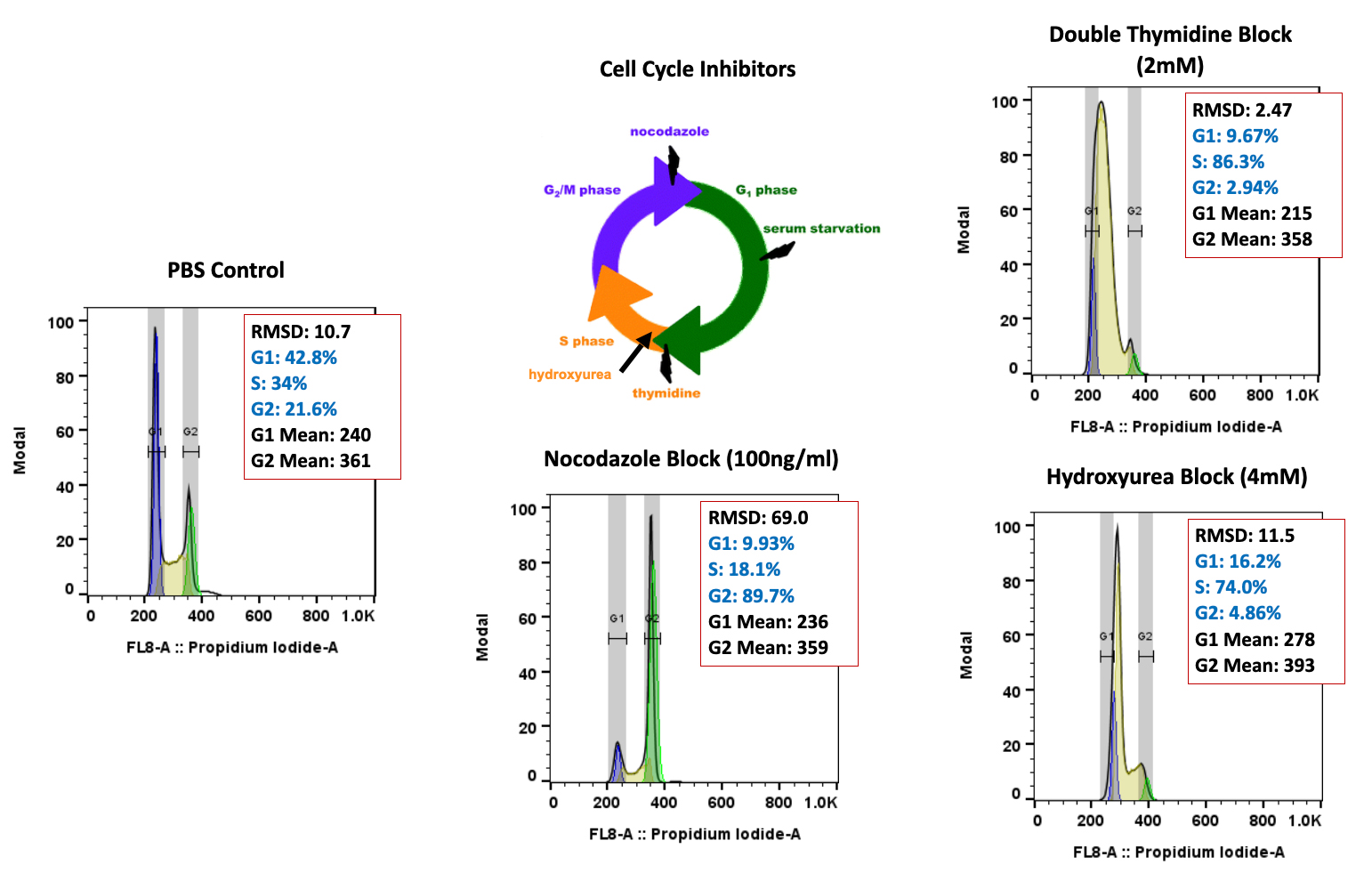
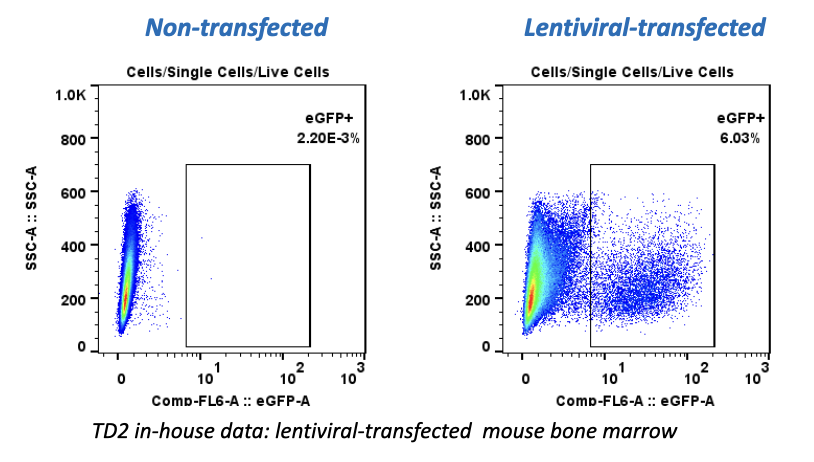
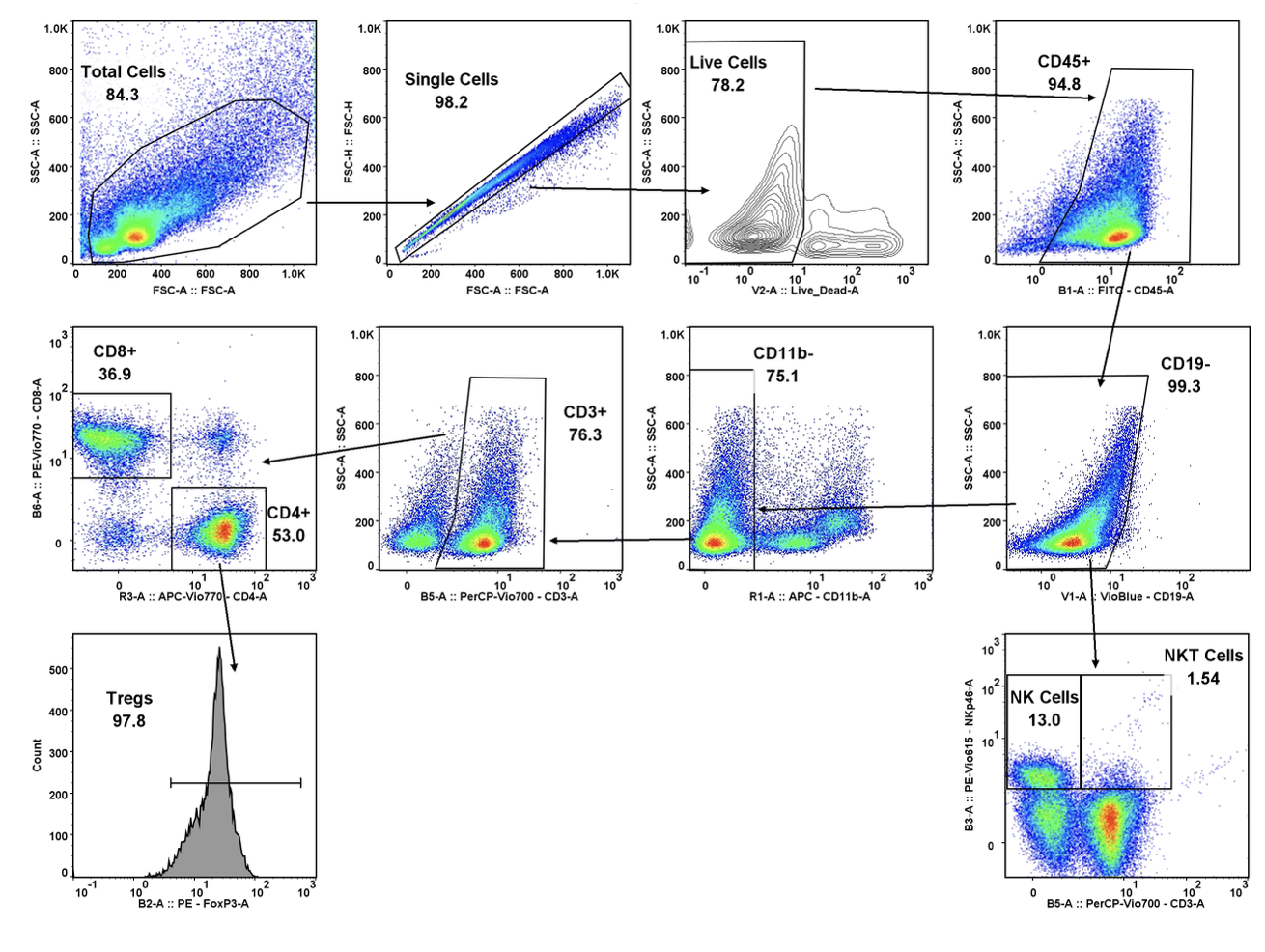
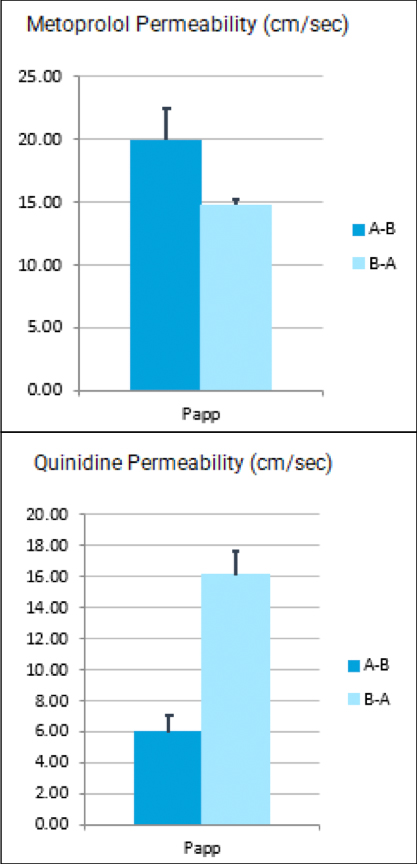
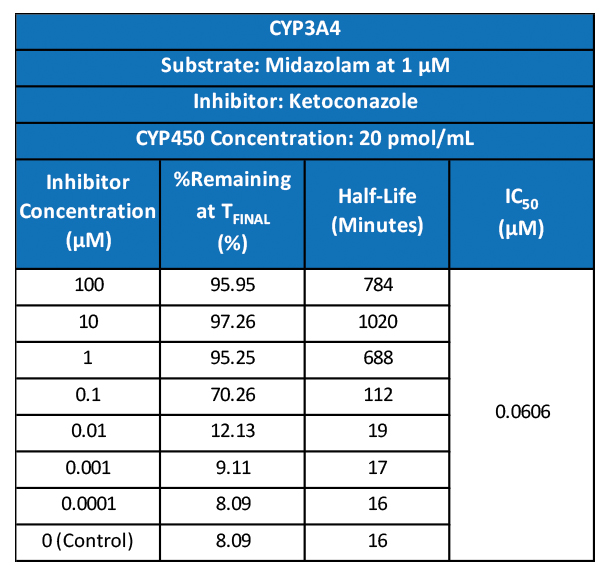
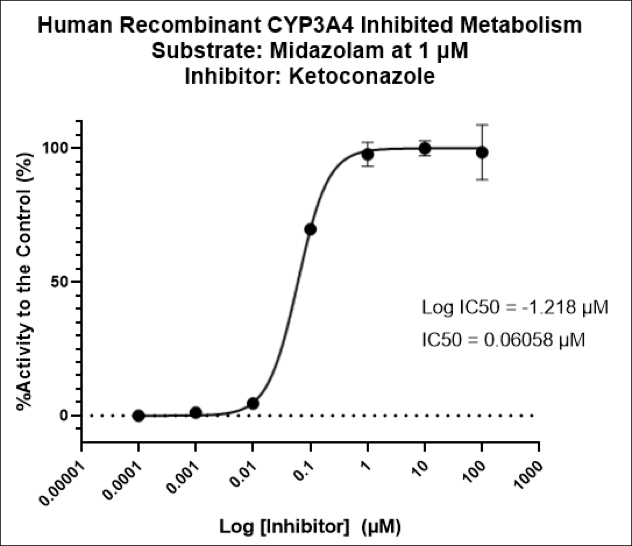
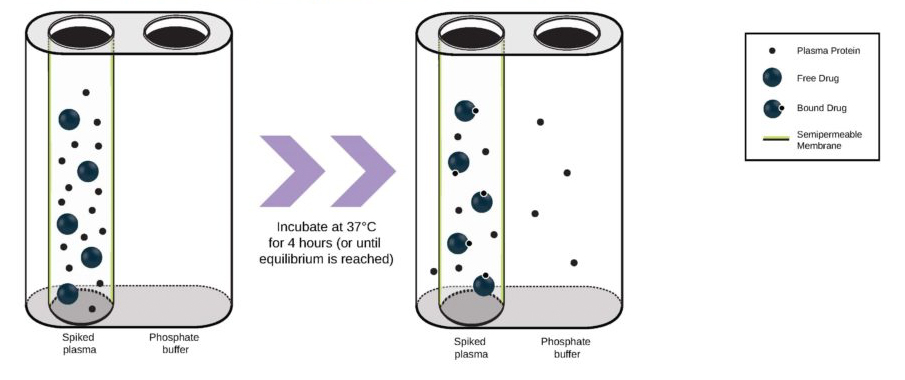
It can be hard for drug makers to pull the plug on a drug in development, even when all signs indicate they should – resulting in many drugs getting past the preclinical floodgates, only to fail. The mistake: Not having a clinical strategy from the get-go.
A smart, defined clinical strategy sets objective expectations at the beginning of a drug’s development for when and on what criteria development will be terminated. This forces drug developers to ask, “Why are we creating this drug in the first place?”
The answer—and the true drug development opportunity—lies in an understanding of the best available science and patient target(s) for the drug.
Before you take your drug to the point of no return, learn more about TD2’s insight from president and CEO, Stephen Gately, on how to be more strategic from the start in Applied Clinical Trials.