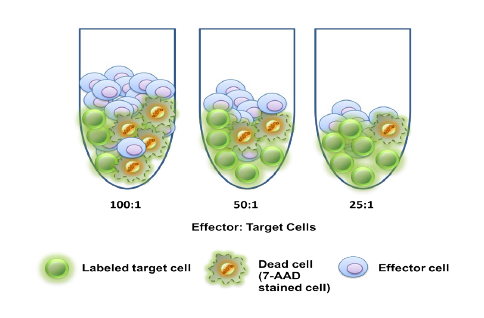
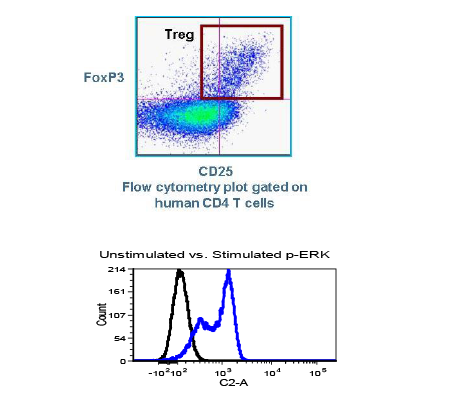
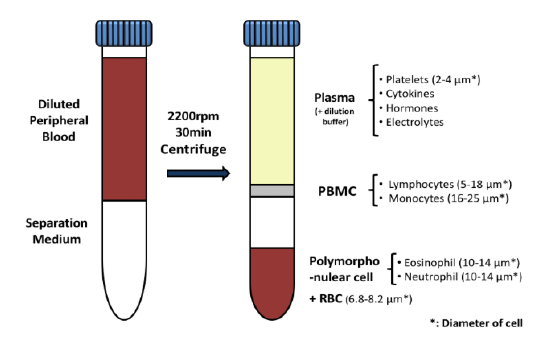
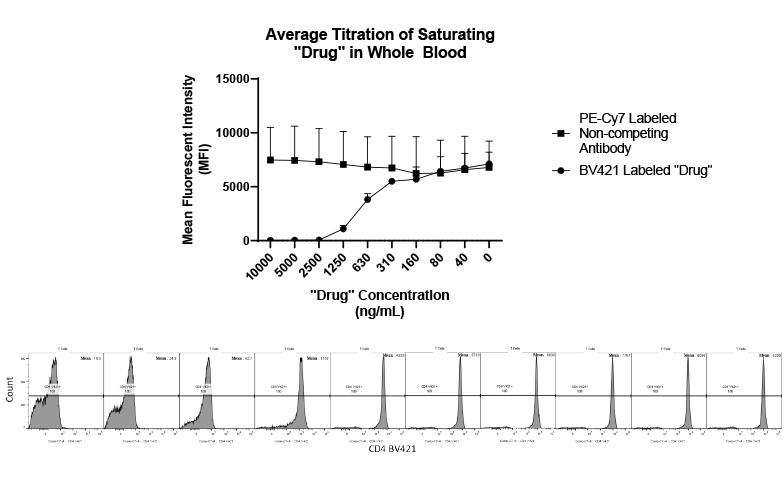
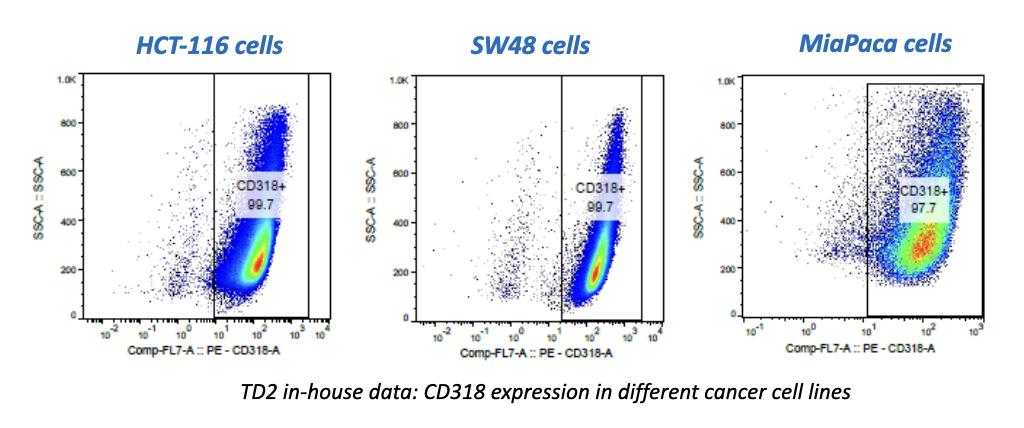
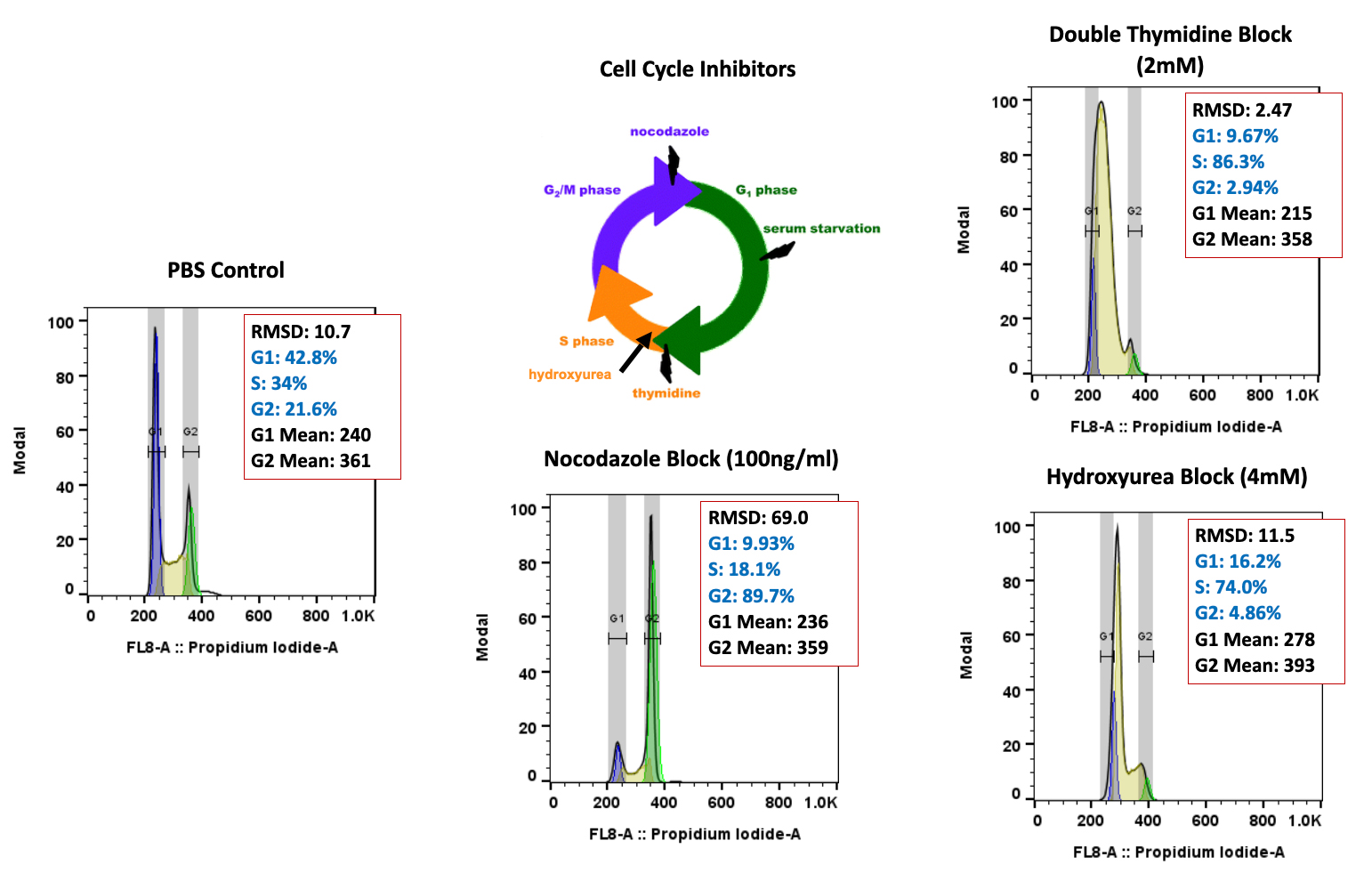
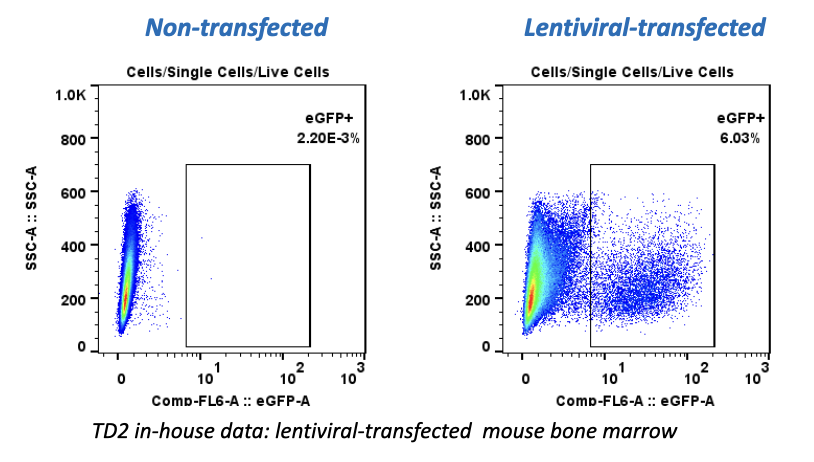
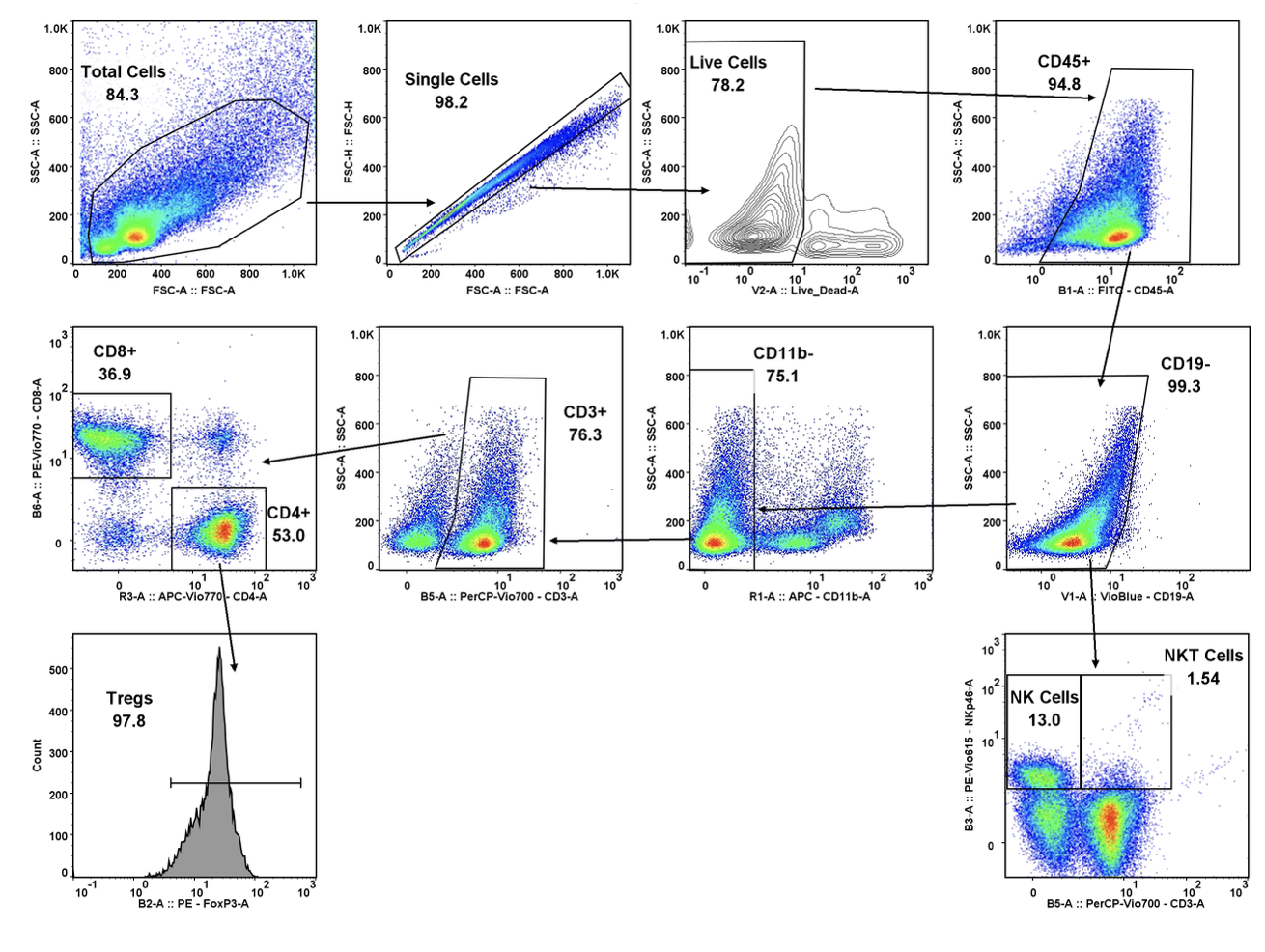
Many emerging biopharmaceutical companies hire contract research organizations (CROs) because of limited staffing and regulatory experience to help them navigate the regulatory pathway. CROs are instrumental in helping these biopharma overcome obstacles such as clinical trial timeline delays, out-of-scope change orders and cost overruns, especially for smaller biotech companies who cannot afford drastic missteps.
All of the above highlight the need to find a CRO that will be the right fit and consistently responsive to clients’ needs.
To propel the biotech companies we partner with, TD2 utilizes a “value-add” approach, where we strive to supplement their capabilities while filling in the gaps in the internal infrastructure to help develop their plan and process every step of the way.
Hear from TD2 president and CEO, Stephen Gately, and chief operating officer, Tara Frank, about how TD2 uses their suite of capabilities to maximize the CRO and emerging biopharma relationship in this recent Applied Clinical Trials article.