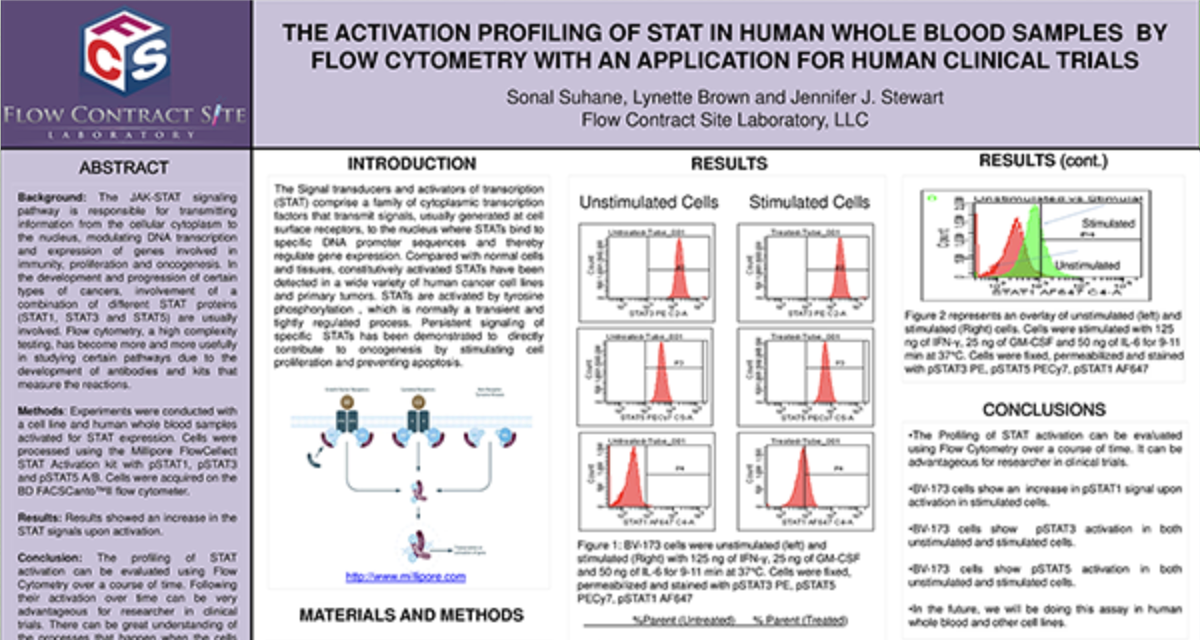
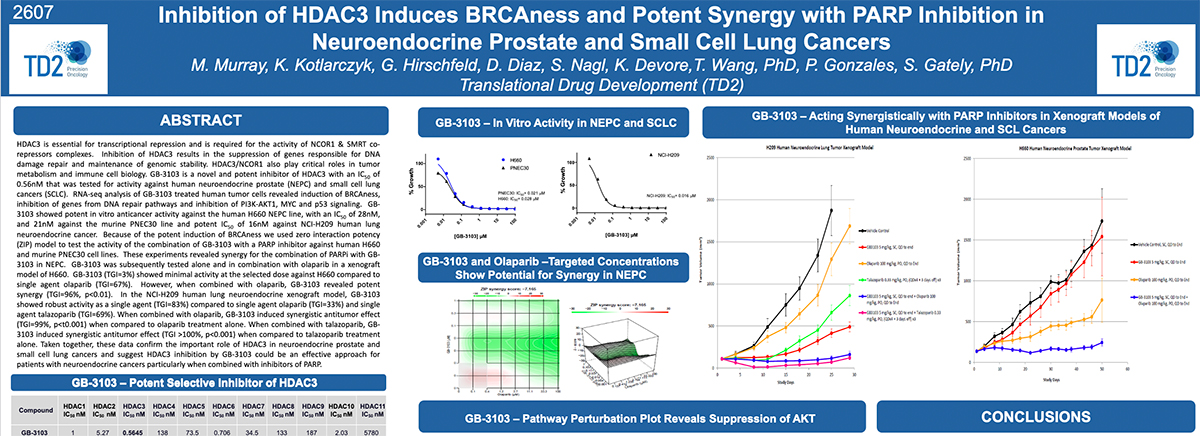
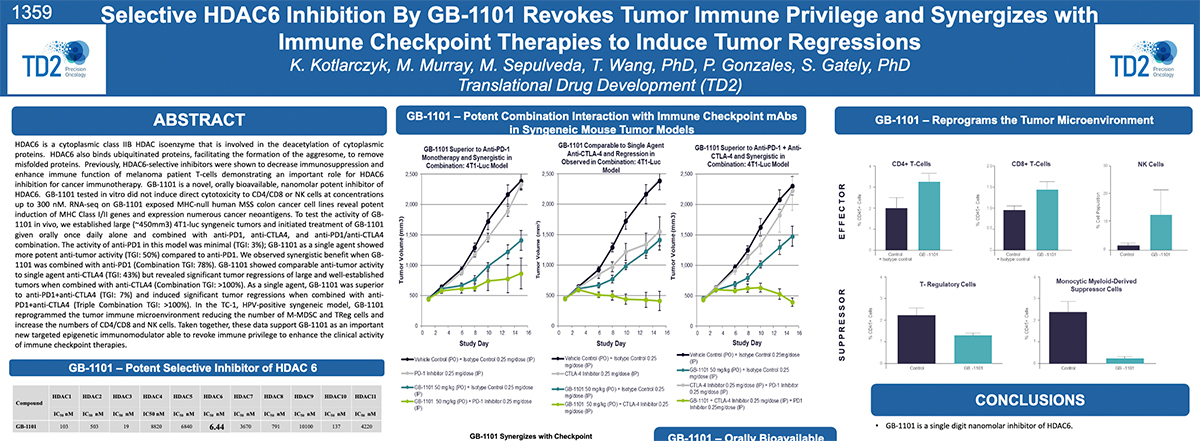
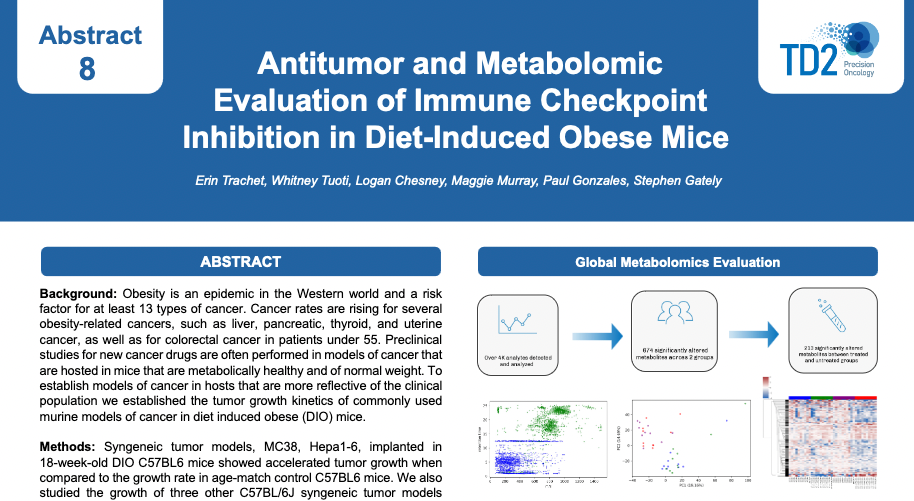
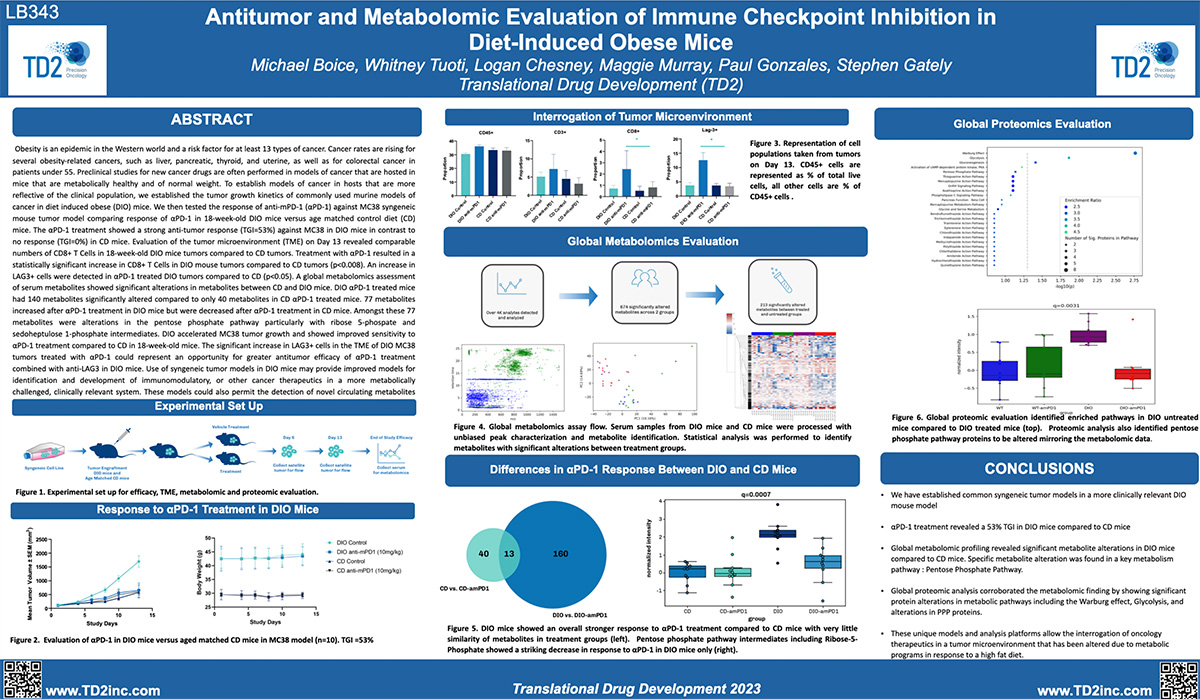
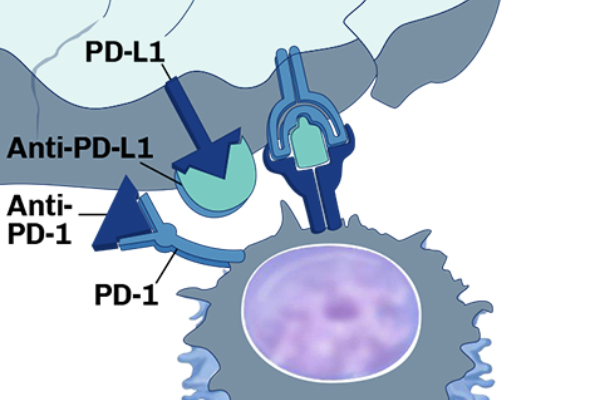
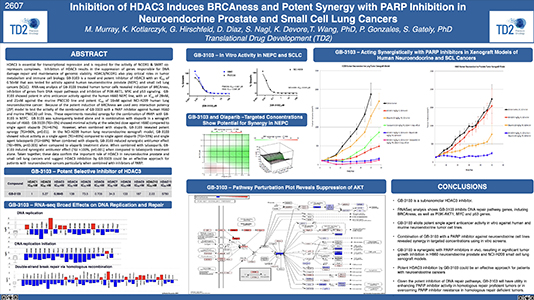
Preclinical
In Vivo and In Vitro Services to Advance Your Drug Discovery
TD2’s integrated suite of preclinical services provides our clients with data that drives the development of clinical strategy. From the discovery stage to NDA strategy, we offer our clients advanced solutions to move their programs forward.
Our comprehensive service offerings utilized to rapidly move your treatments to the clinic include DMPK/ADME, in vitro and in vivo pharmacology, expert study design and execution. Your in vivo pharmacology studies are run in our AAALAC-accredited rodent facility with the highest regard for animal welfare. All studies are conducted with a commitment to meeting the high-quality standards you expect, and each offering is customizable to meet your unique development needs. With expert drug development insight and strategy and skilled project management, TD2 is your partner for an efficient path to the clinic.
Preclinical
Models and Services
TD2’s service offering is consistently expanding and currently includes:
- Non-GLP Safety/Tolerability
- Syngeneic Tumor Models
- In vitro Pharmacology Assays
- Orthotopic Tumor Models
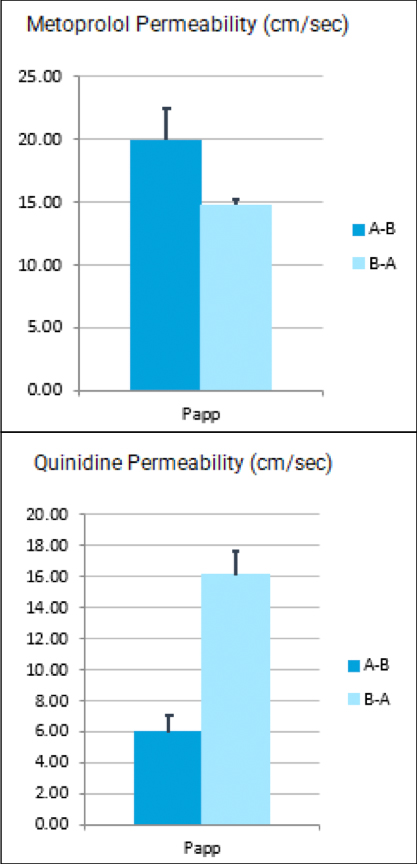
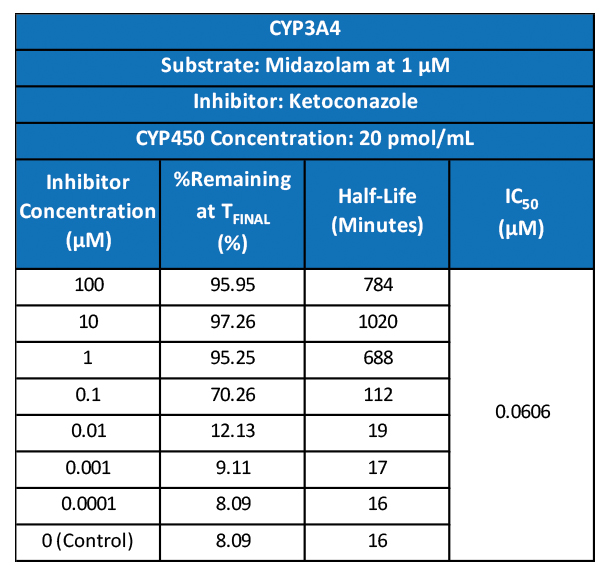
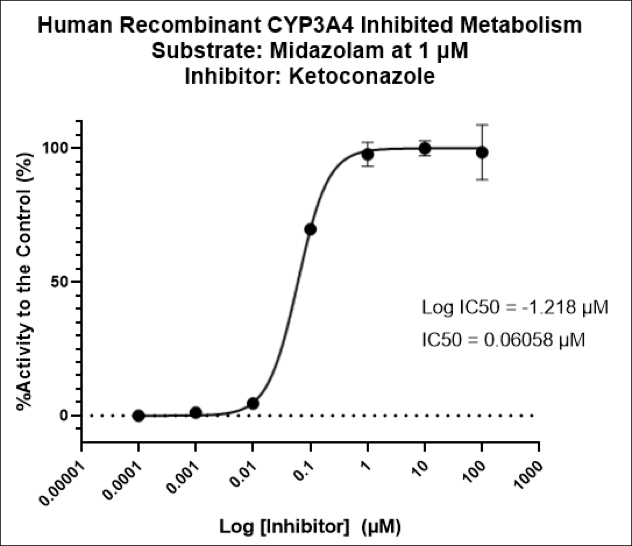
- DMPK/ADME services
- Adoptive Cell Therapy Models
- Tumor Xenograft Models
- In vivo Optical Imaging
- Humanized Mouse Models
- Patient-derived tumor xenograft models (GBM)
- Diet-Induced Obesity Tumor Model
- Metabolomics and Proteomics Analysis
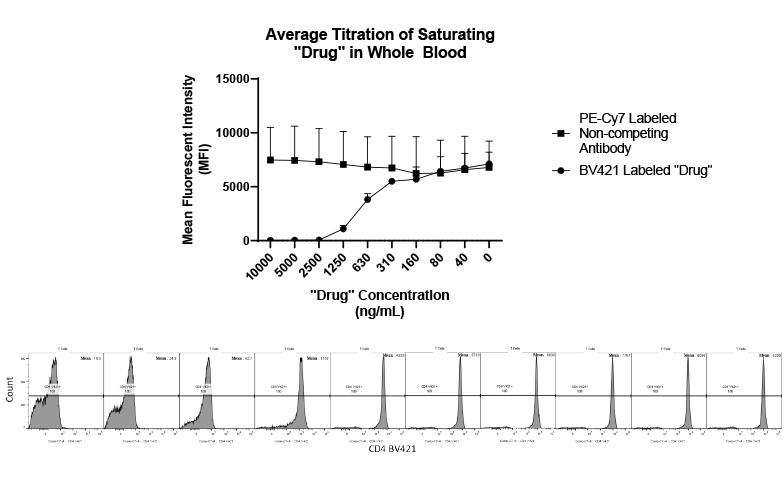
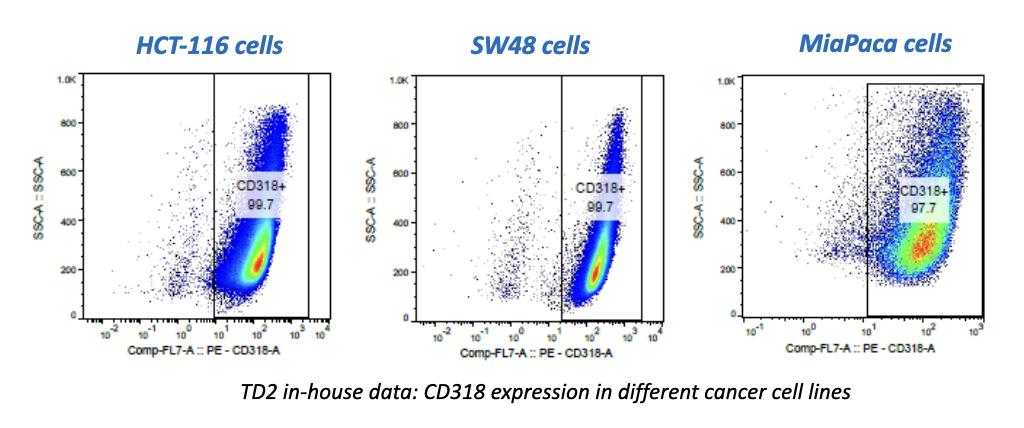
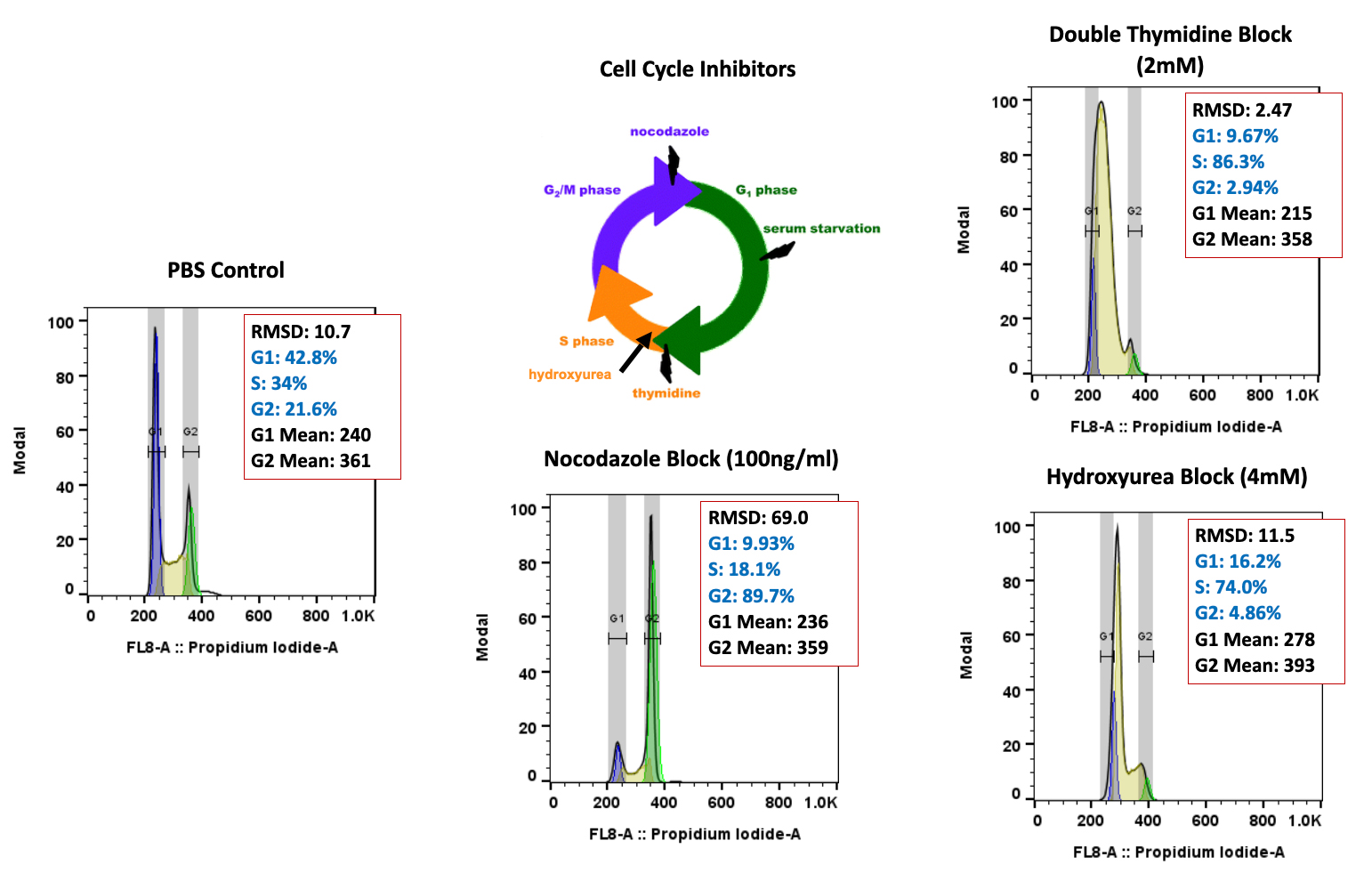
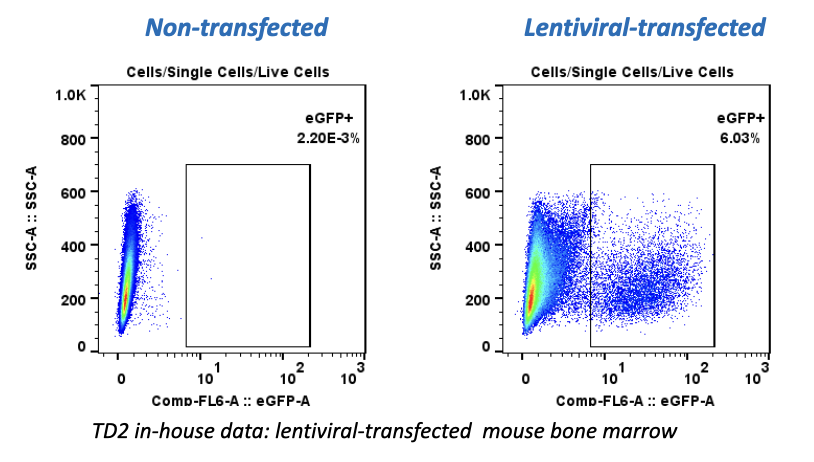
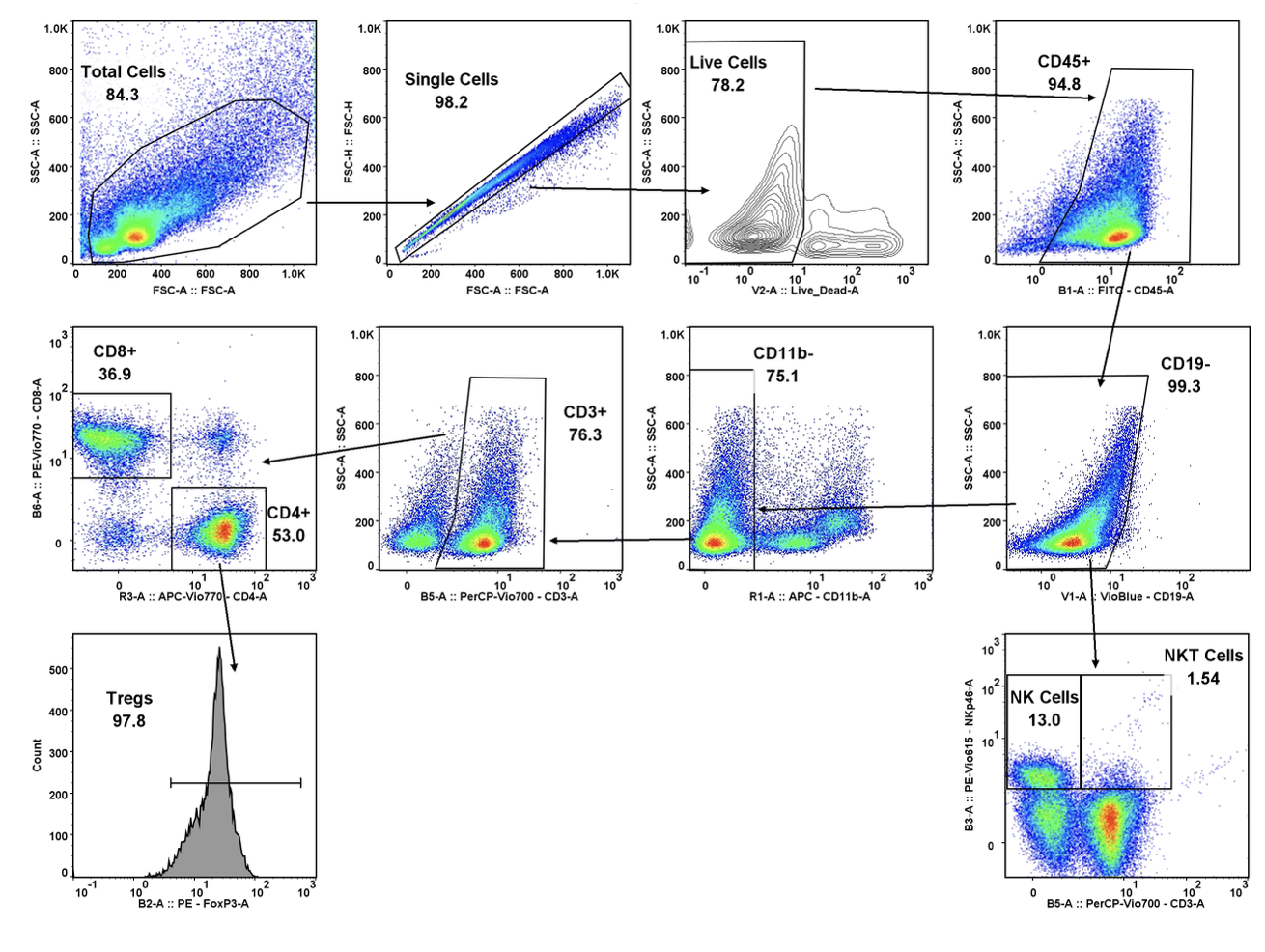
- Flow Cytometry
Tumor Models
Trusted IO Models
At TD2, we are dedicated to providing you with the most comprehensive preclinical immuno-oncology studies possible. Our experienced team offers a broad range of human and murine tumor models that allow for the planning and execution of studies in solid or hematologic malignancies.
Our syngeneic tumor models are fully characterized for gene expression, TIL baseline populations, and response to common immune checkpoint inhibitors (ICI), allowing for rapid, trusted study outcomes. These syngeneic cell lines can also be combined with our wide range of humanized immune checkpoint inhibitor mouse models to evaluate your ICI treatment. When syngeneic mouse systems are not applicable for your drug, we can employ the use of humanized mice either by engrafting with human PBMCs or other effector cells or utilizing sourced CD34+ humanized mice.
TD2’s vast experience in Adoptive Cell Transfer Therapy studies in both solid and hematologic tumors is unmatched in the industry. We have the expertise to provide you with the most accurate and reliable data possible, ensuring the success of your drug development program.
Cell Therapy Models +
Syngeneic Models +
Humanized Immune Checkpoint Inhibitor Mouse Models +