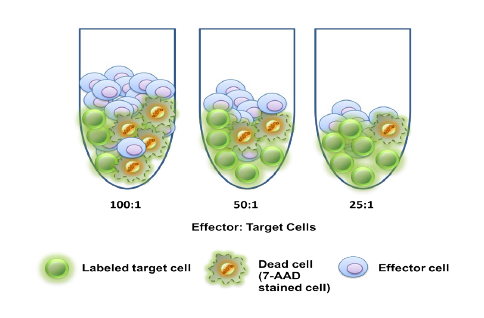
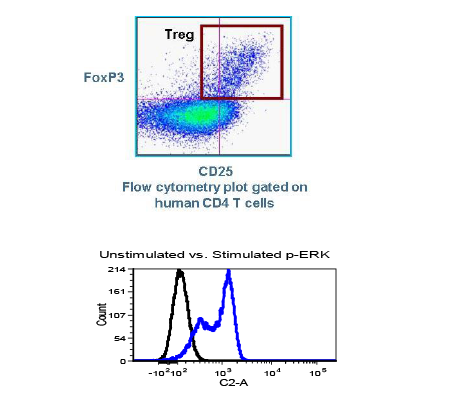
Translational Drug Development (TD2)—an oncology-specialty CRO that guides medicines through the preclinical, regulatory and clinical trial processes—conducted preclinical trials over the past six months that examined the effectiveness of a durable, off-the-shelf anti-CD70 CAR T cell against a human clear cell renal carcinoma tumor xenograft model. Results of the studies, performed in partnership with the study sponsor CRISPR Therapeutics, were presented at the AACR Annual Meeting in Chicago on April 16, 2018.
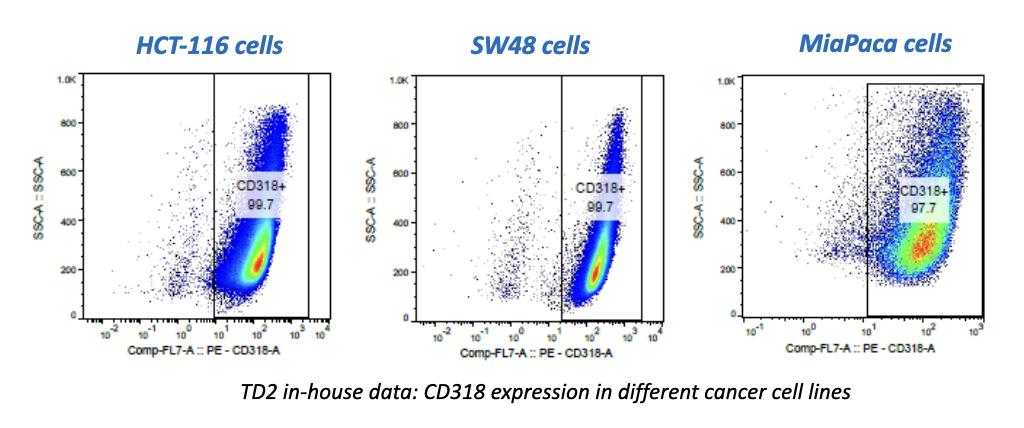
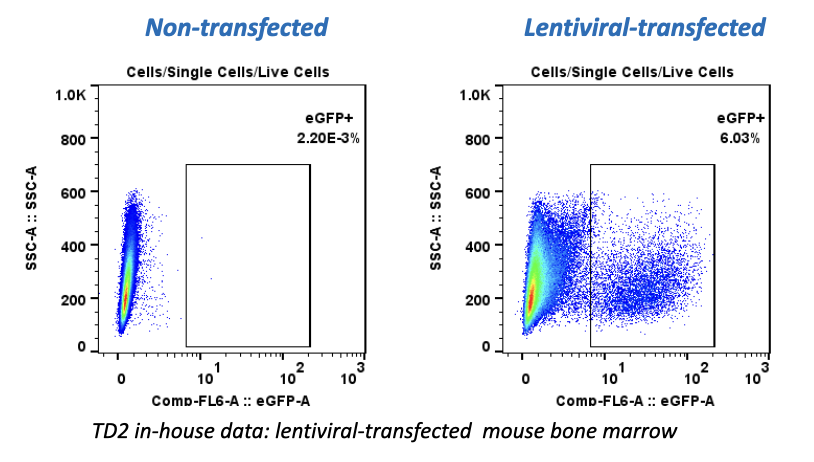
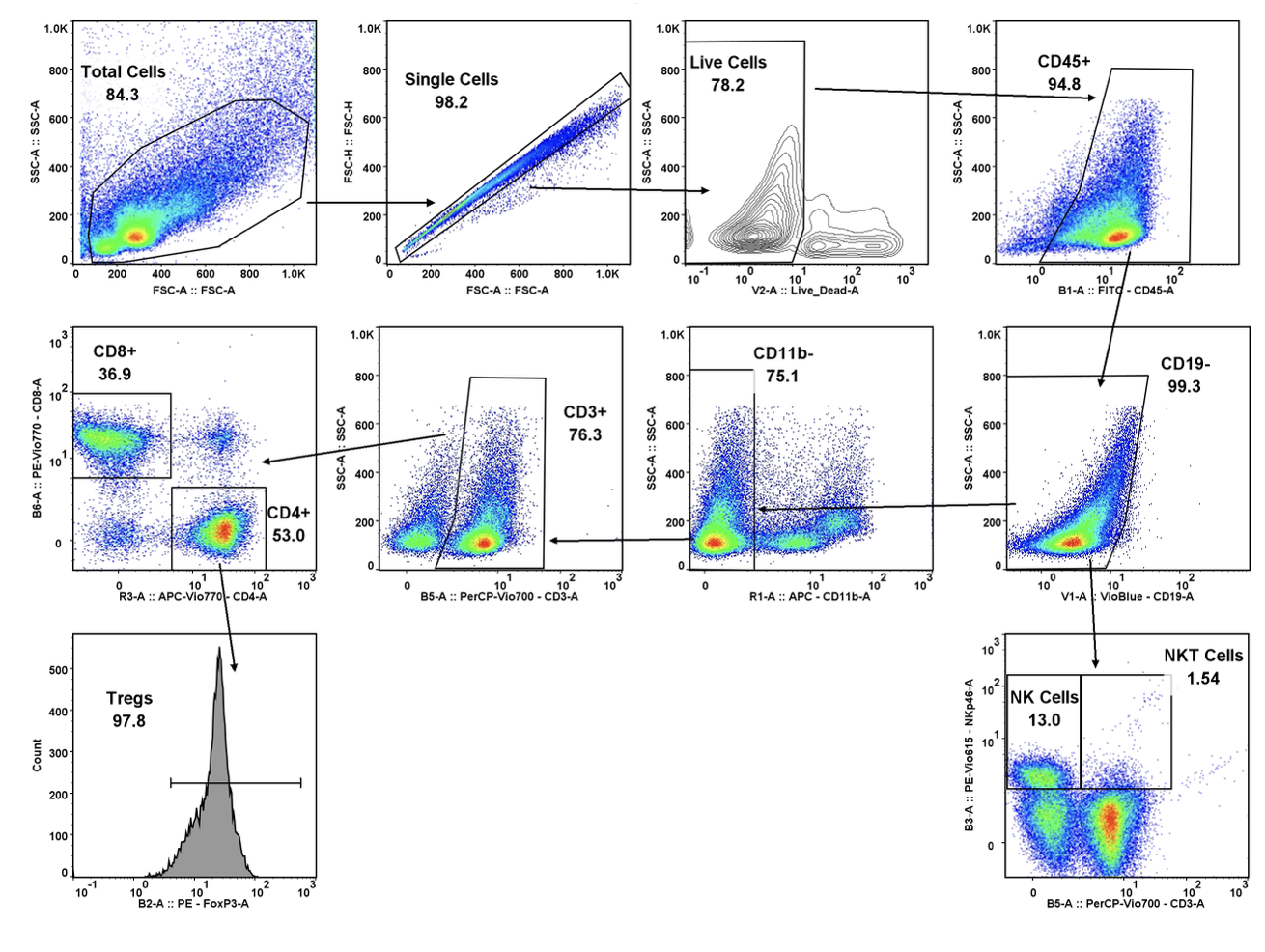
While there have been recent commercial approvals of chimeric antigen receptor (CAR) T cells to treat leukemia and lymphoma, there is limited preclinical and clinical activity reported for CAR T cells directed at solid tumor antigens. To address this, CRISPR Therapeutics—a biopharmaceutical company focused on developing transformative gene-based medicines for serious diseases—developed allogeneic CAR T cells targeting the CD70 antigen. CD70 is expressed in both hematologic malignancies and solid cancers, including renal cell carcinoma (RCC). The company selected TD2 as its preclinical partner to conduct these important trials.
“CRISPR Therapeutics’ novel approach to targeting solid cancers is leading to game-changing results in this emerging and transformative area of oncology research,” said Paul Gonzales, vice president of nonclinical operations for TD2. “We’re honored to carry out studies to support the technology developed at CRISPR Therapeutics and look forward to advancing CAR T therapies for patients with cancer.”
Ongoing Trends in CAR T Cell Research
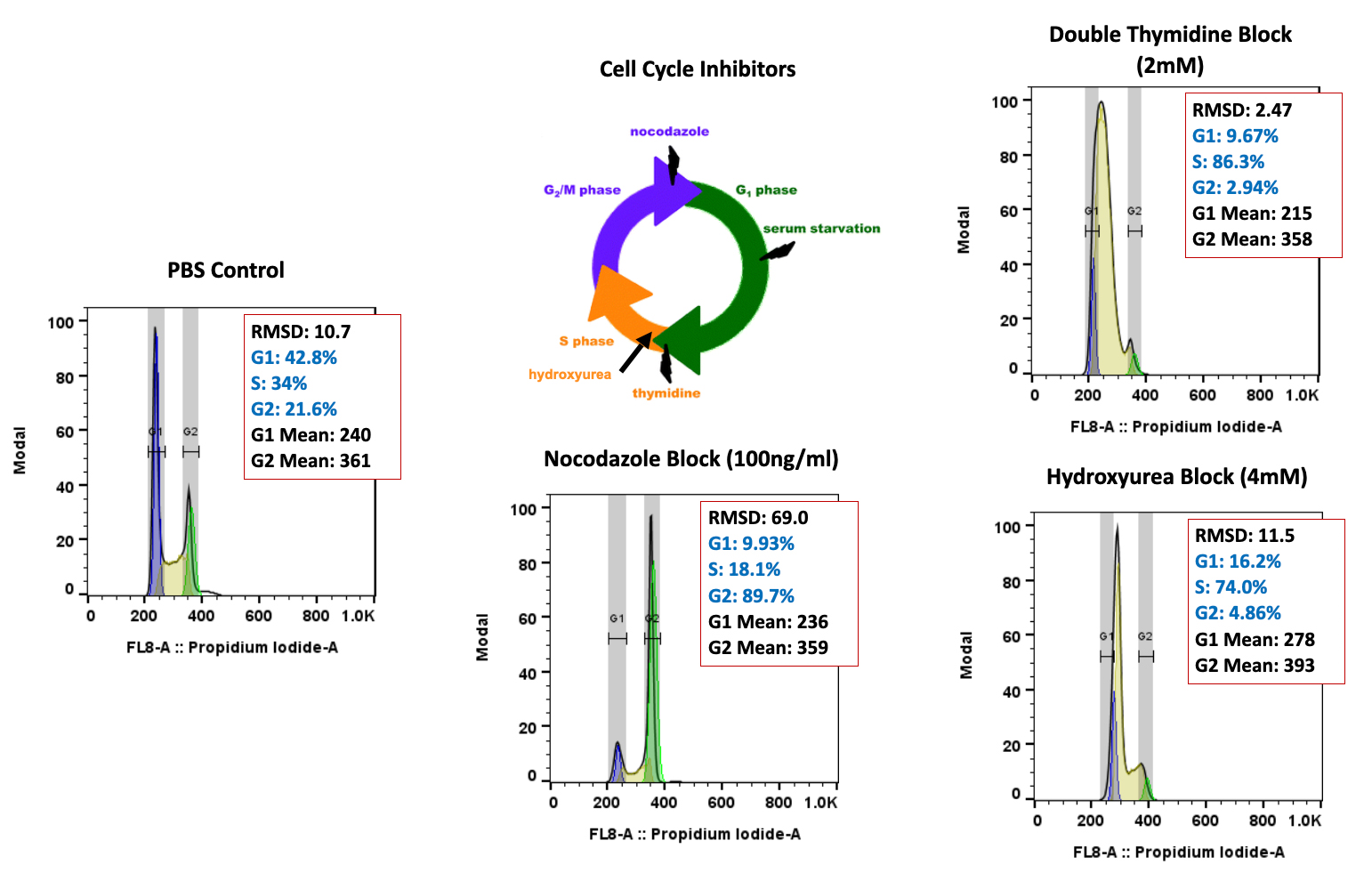
The research reflects continued interest in adoptive T cell transfer (ACT) to mediate antitumor effects in otherwise treatment-refractory cancers. Three forms of ACT are being developed for cancer therapy: tumor-infiltrating lymphocytes (TILs), T cell receptor (TCR) T cells and CAR T cells. CAR T cells were recently named “Advance of the Year” by the American Society of Clinical Oncology (ASCO) in its 2018 annual report.
“This is an area that has been celebrated as a breakthrough technology and while it comes with significant benefits to patients, it is not without equally significant challenges,” said Stephen Gately, Ph.D., Chief Executive Officer of TD2. “We see this as an area of high interest over the coming years as companies continue to innovate to overcome these challenges and improve patient outcomes. As a CRO committed to our clients’ goals, we’re excited to explore and make available new model systems and techniques that contemplate clinically relevant scenarios and combination therapies that will reduce toxicities and make responses seen in patients more durable.”
TD2 will continue preclinical work with CRISPR Therapeutics to extend these findings involving the CD70 antigen to other cancers.