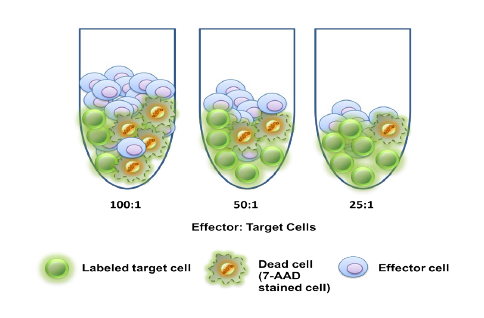
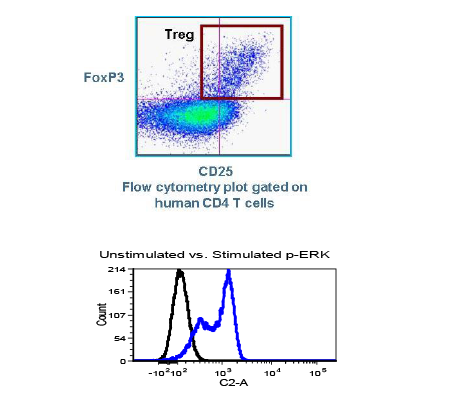
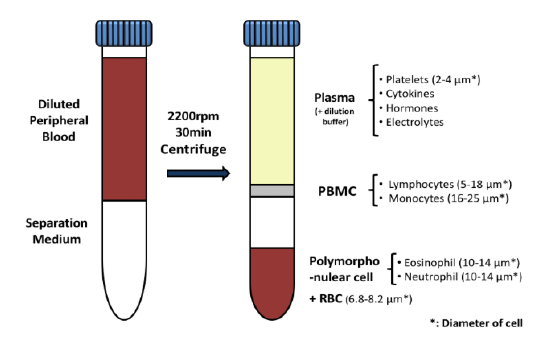
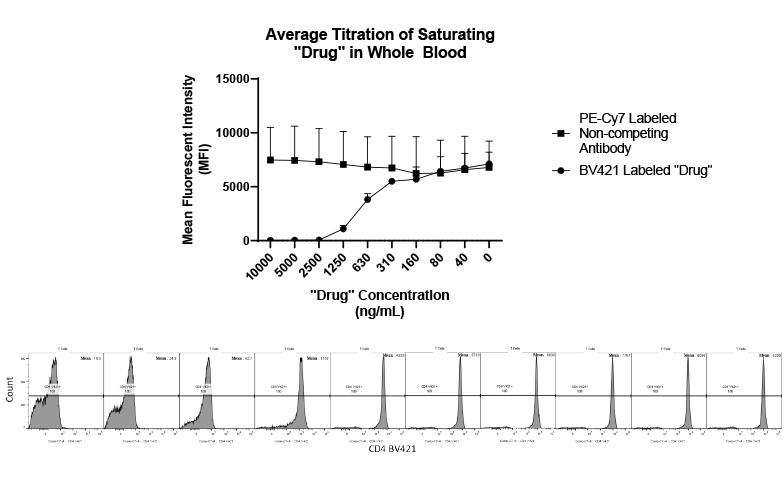
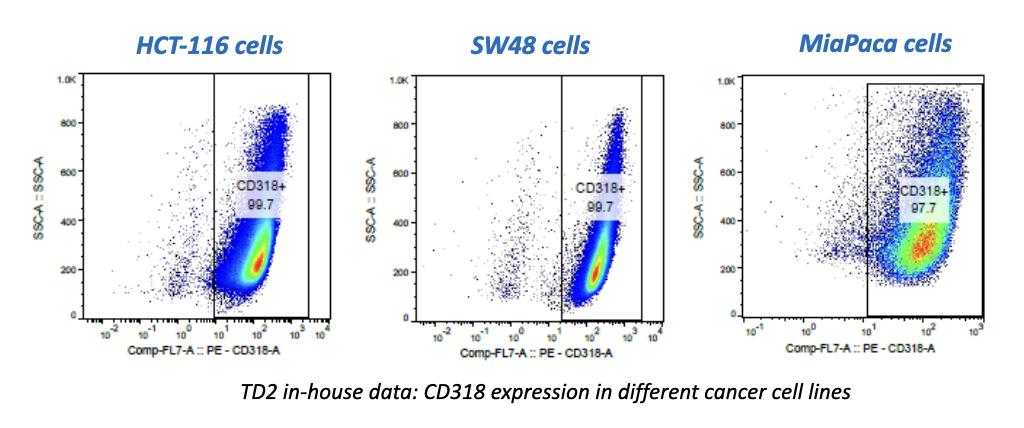
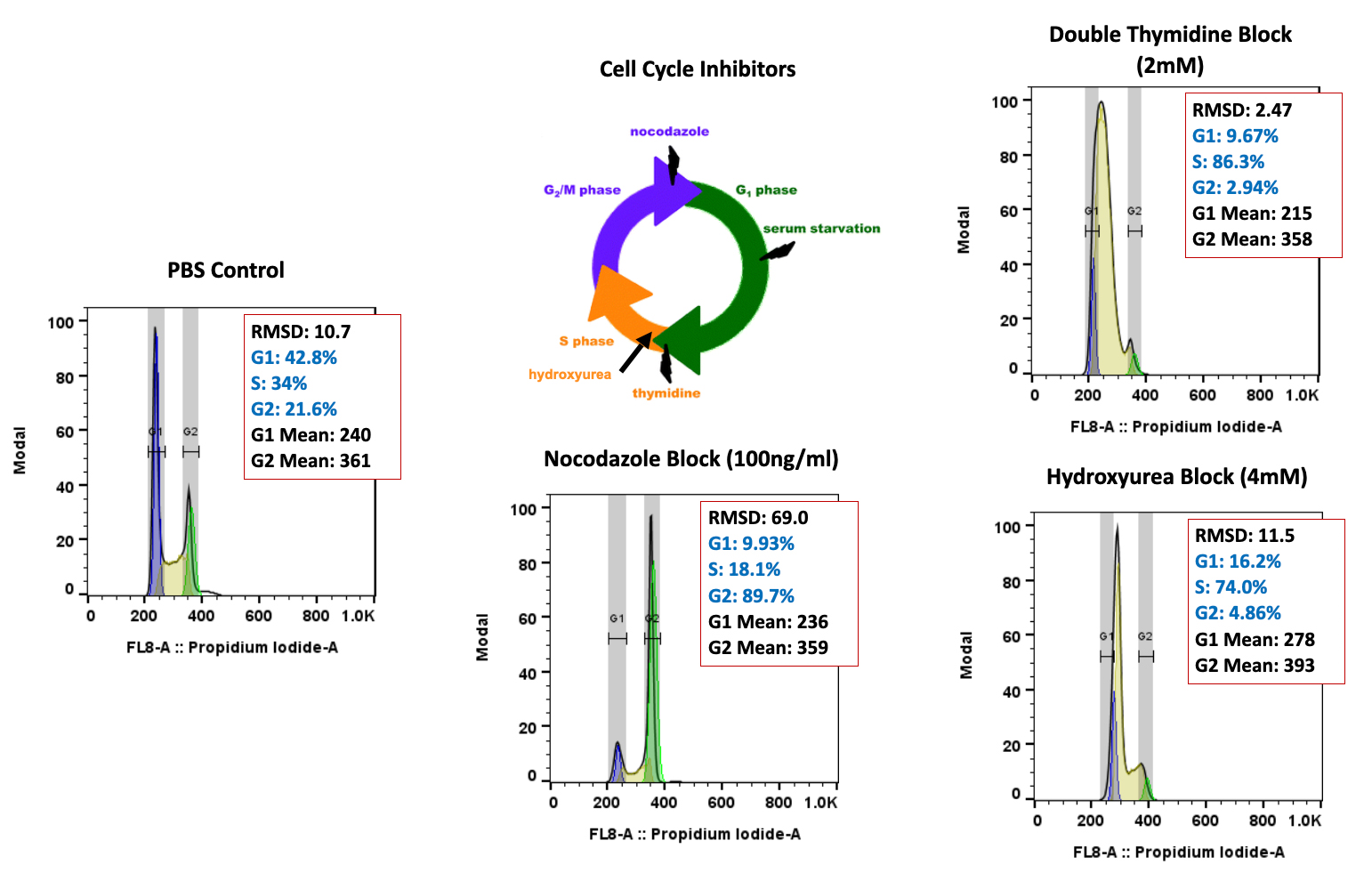
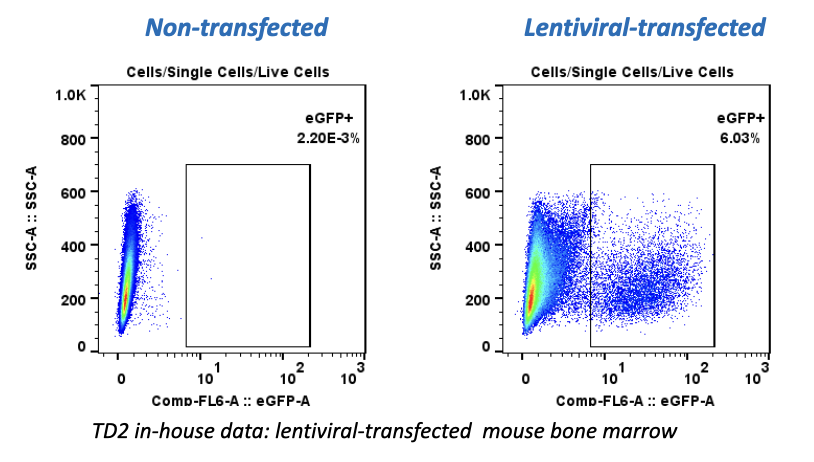
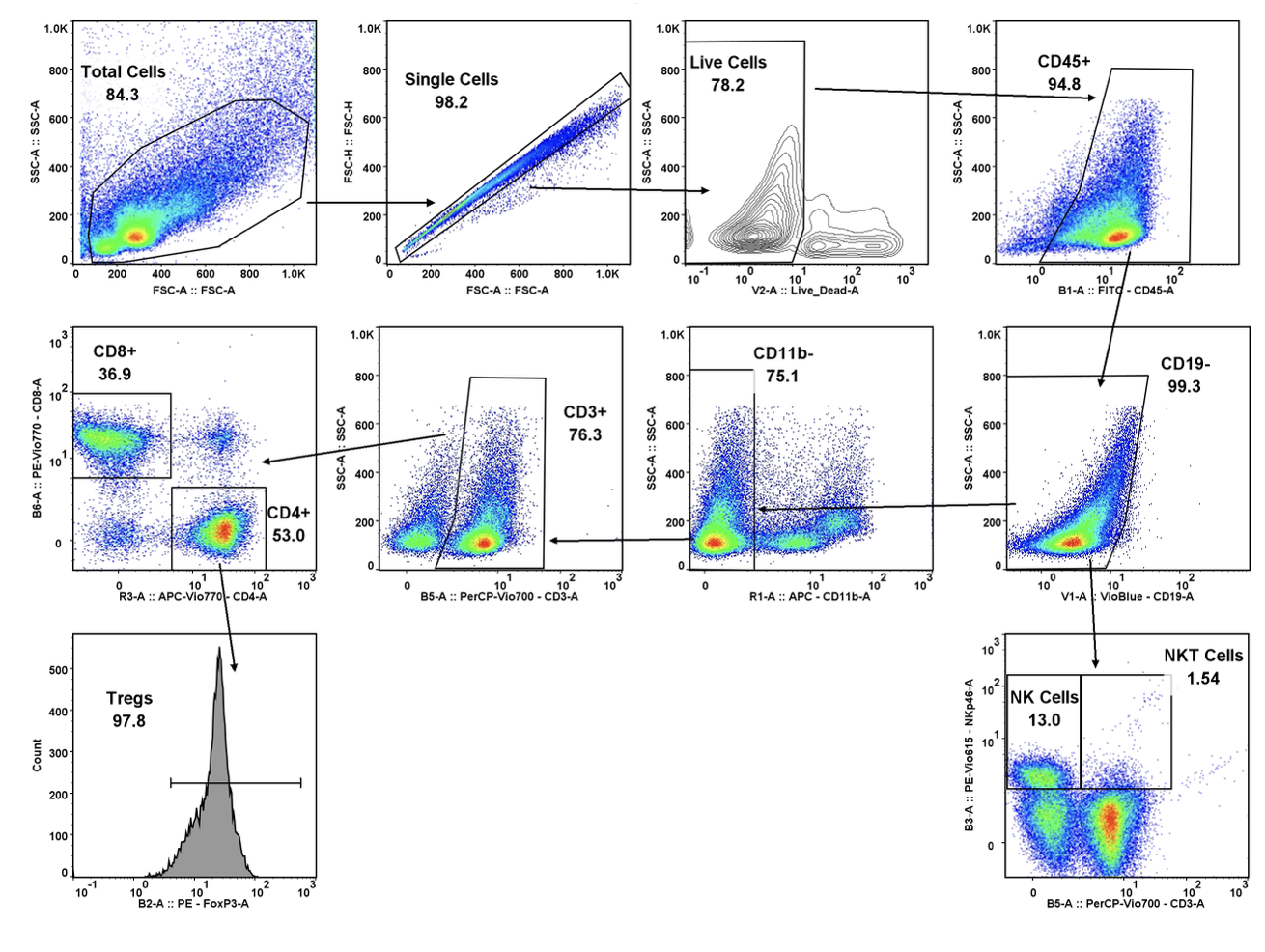
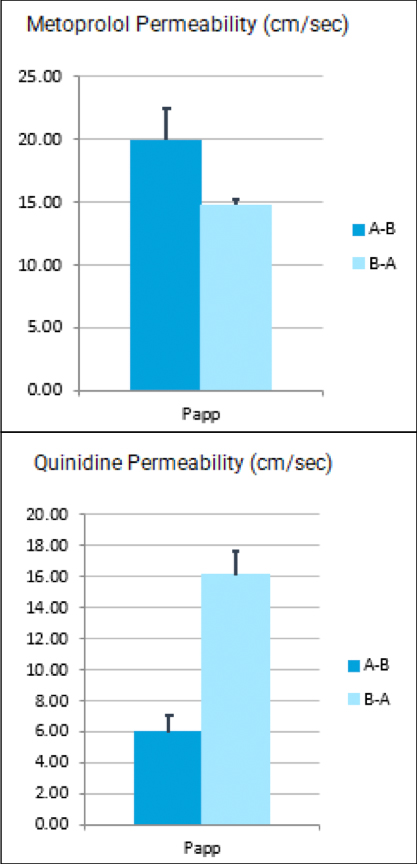
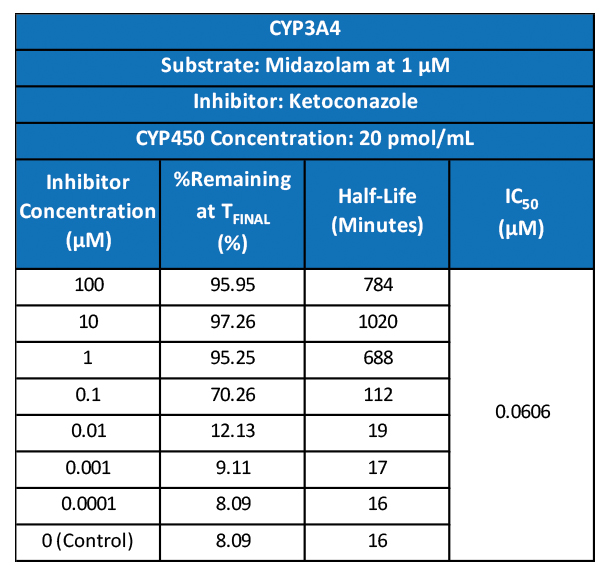
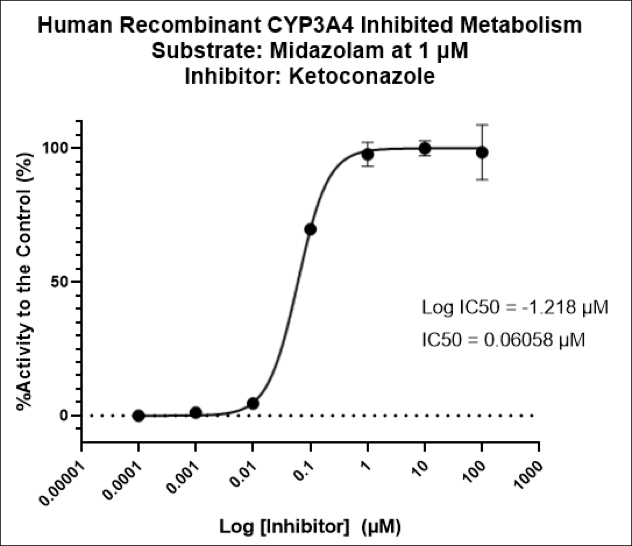
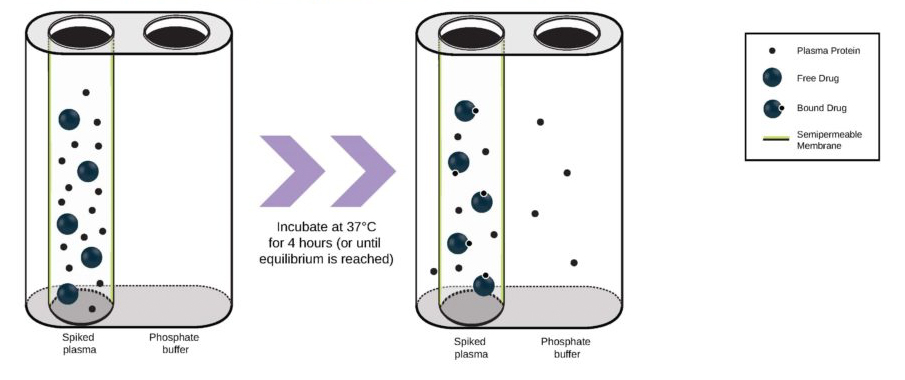
Cancer is now classified not only by the organ of origin, but by genomic and proteomic profiling.
These “omic” contexts allow drug developers to target tailored treatments even to the rarest of cancers. TD2 CEO, Stephen Gately, shares how cancers can be a rare cancers when you consider their various molecular defects, and how drug developers can focus development of new medicines against these unique “omic” contexts in a recent article published in Applied Clinical Trials.
“Precision medicine provides physicians with the opportunity to avoid less effective treatments and focus on rationally selected medicines that improve clinical outcomes for patients,” said Gately.
Read more about Gately’s outlook on the future of precision medicine in cancer treatment research in the full article.