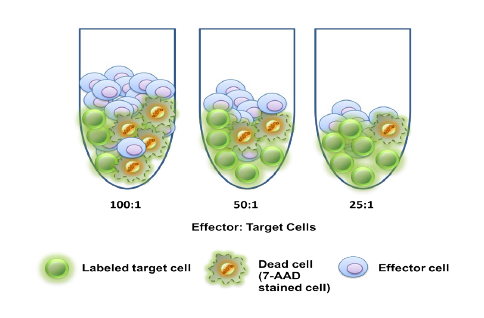
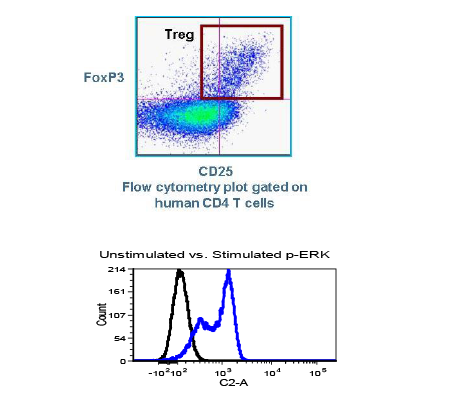
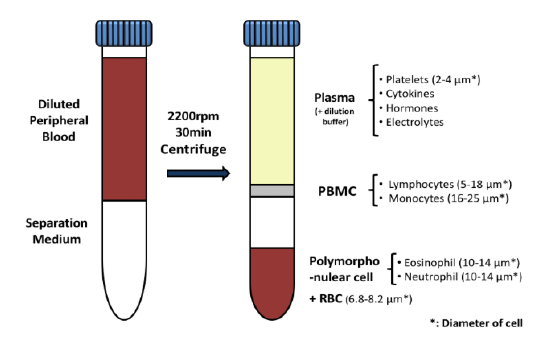
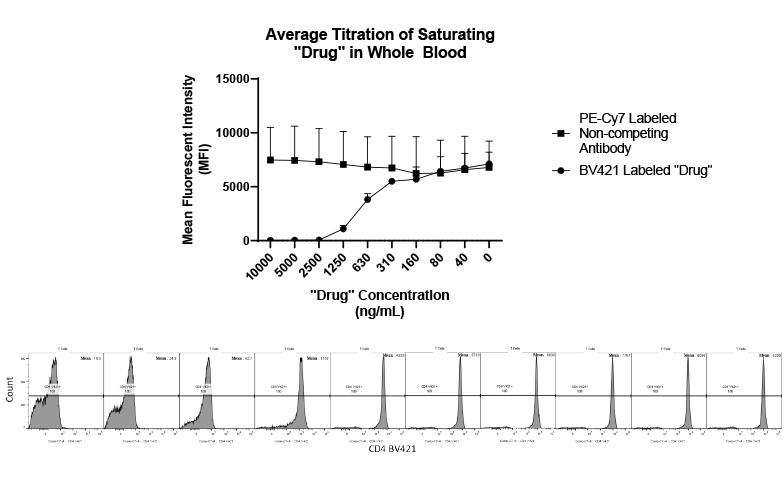
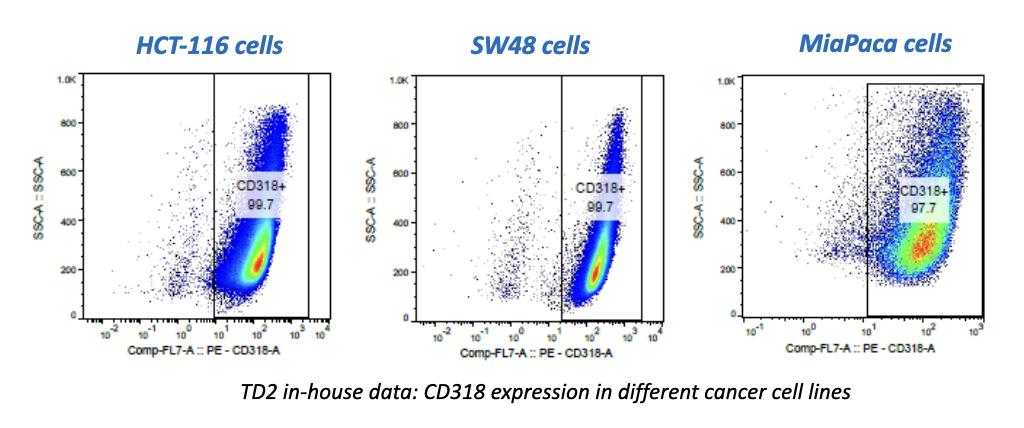
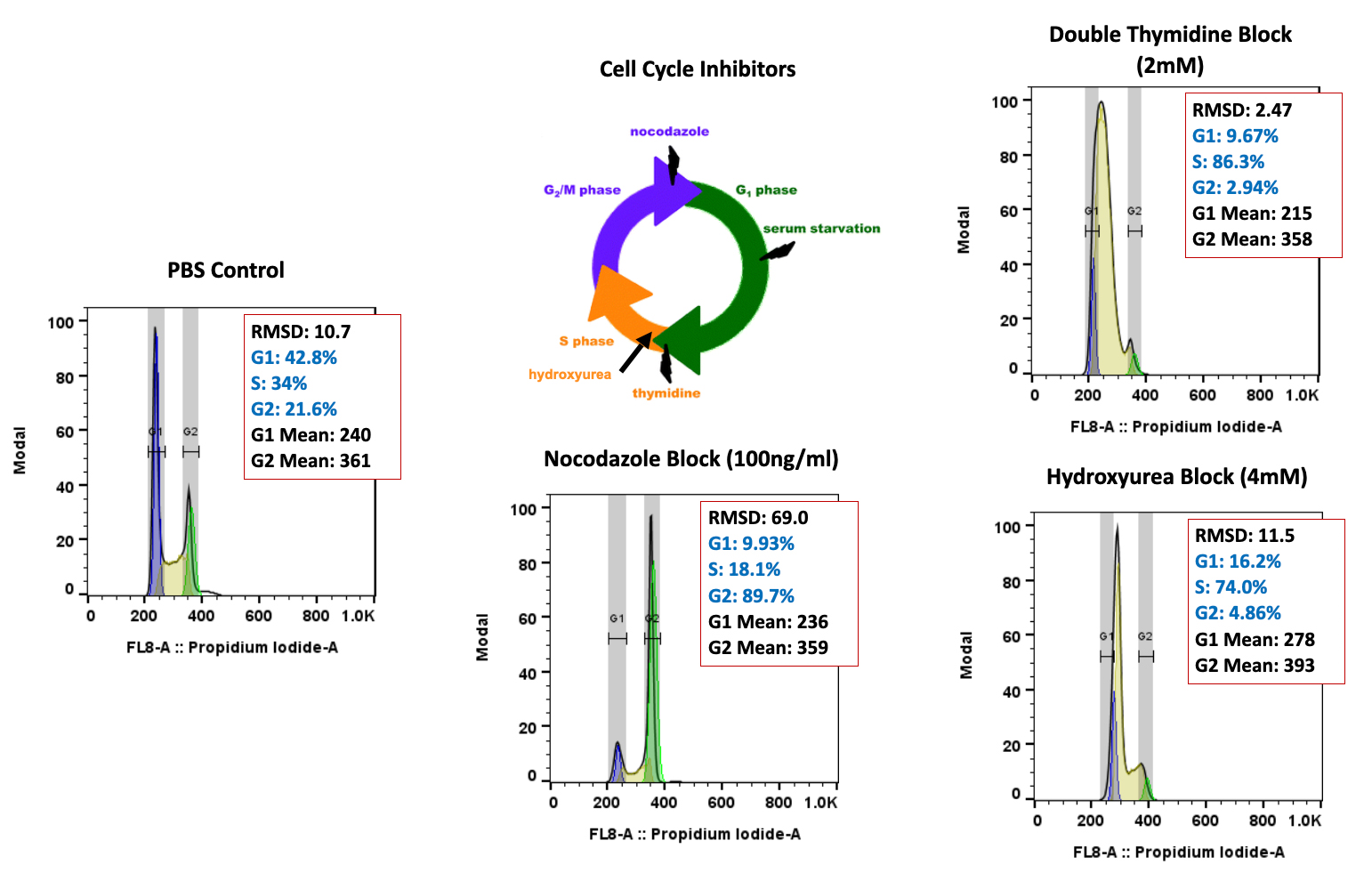
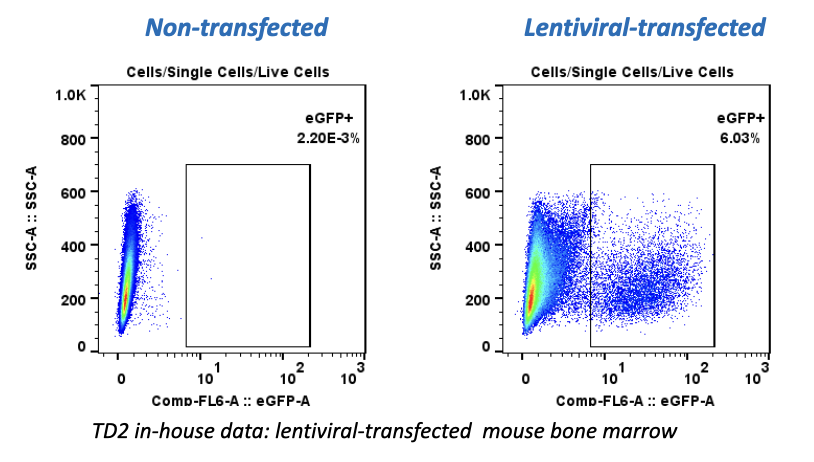
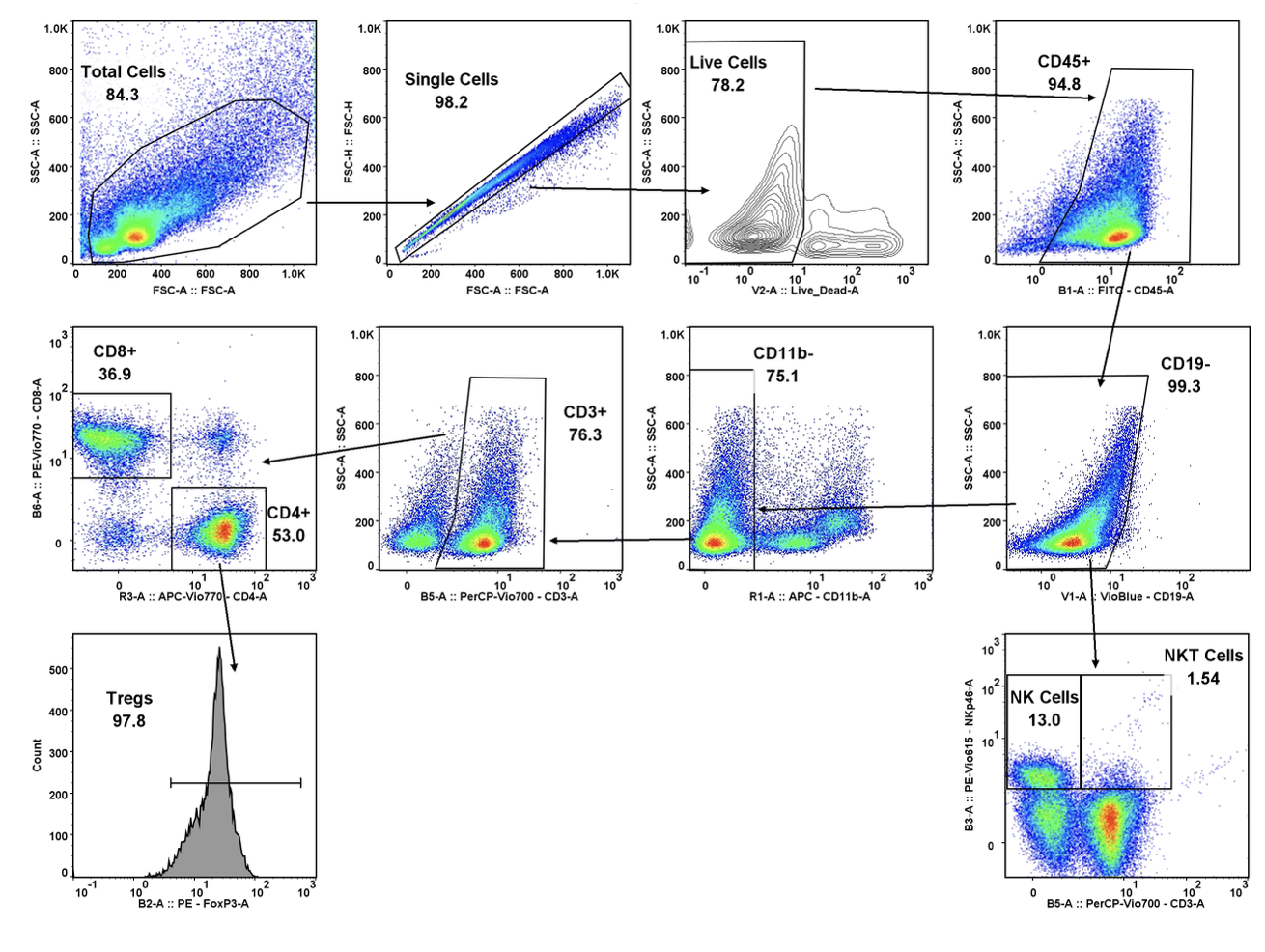
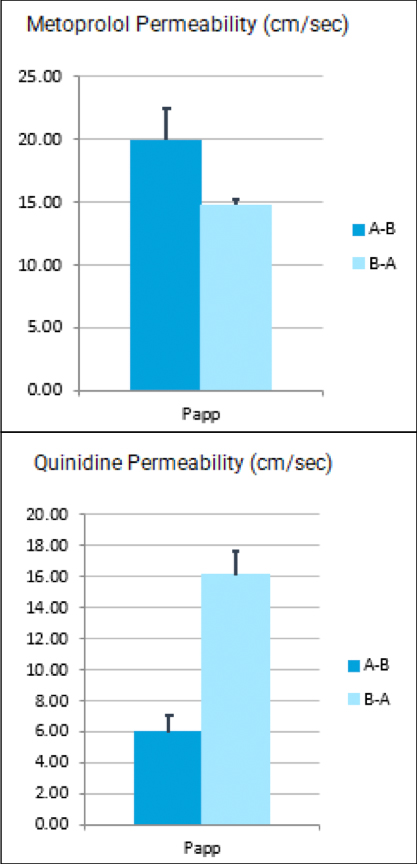
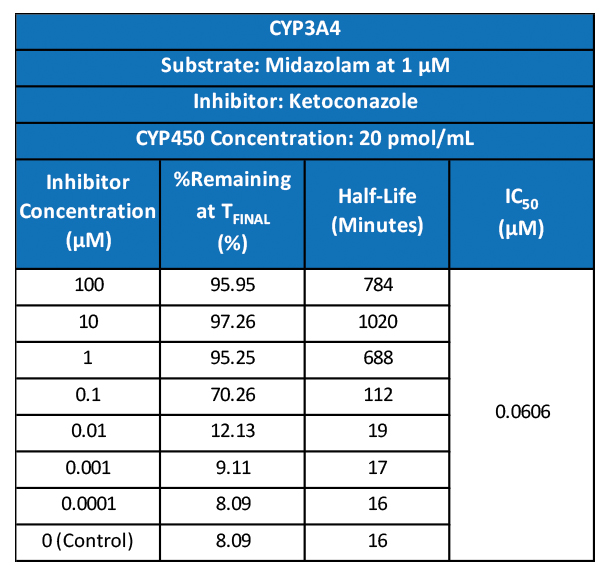
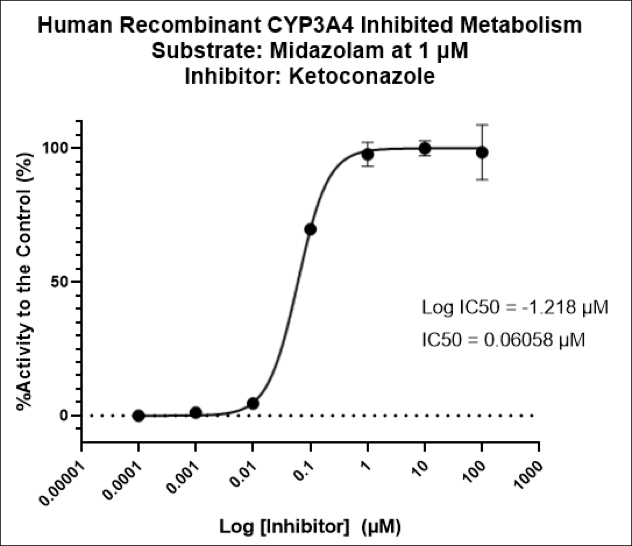
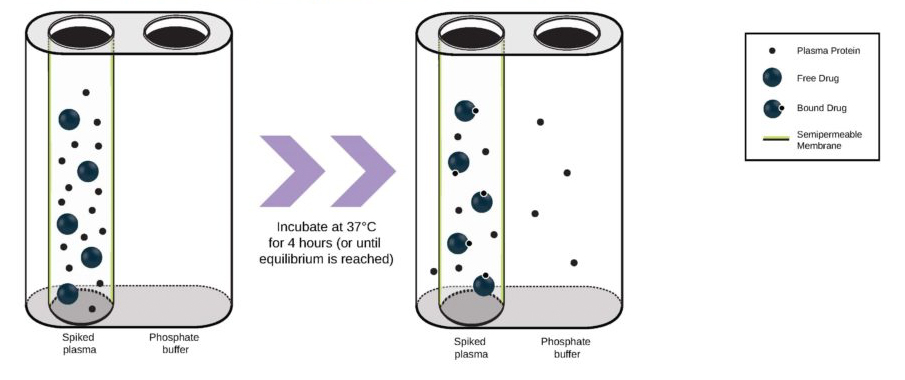
With COVID-19 impacting the conduct of clinical trials, a lot of discussion has been around what changes have allowed trials to continue recruitment. Protocol amendments and the use of technology are just a couple examples of trial aspects that have been drastically impacted by the pandemic, and are expected to see many implemented changes remain.
Read the full article here to learn more about how oncology CROs are uncovering solutions to make trials available to patients now and into the future amid current challenges.