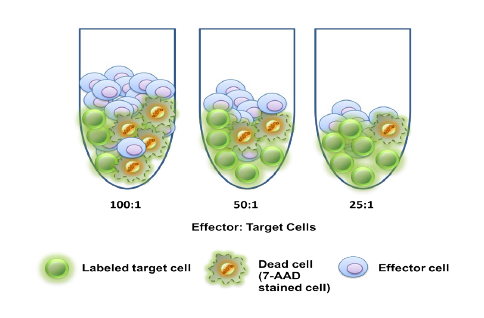
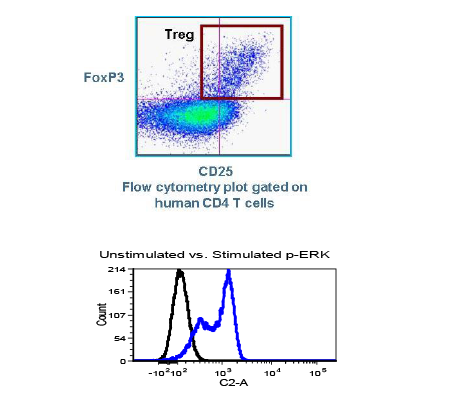
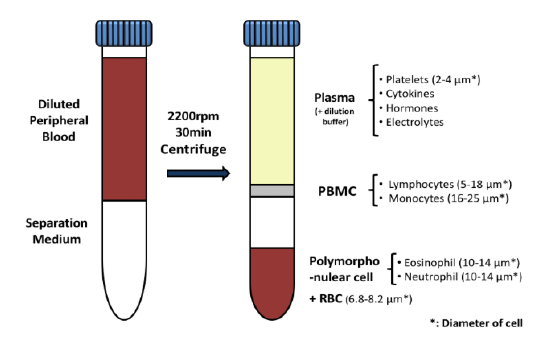
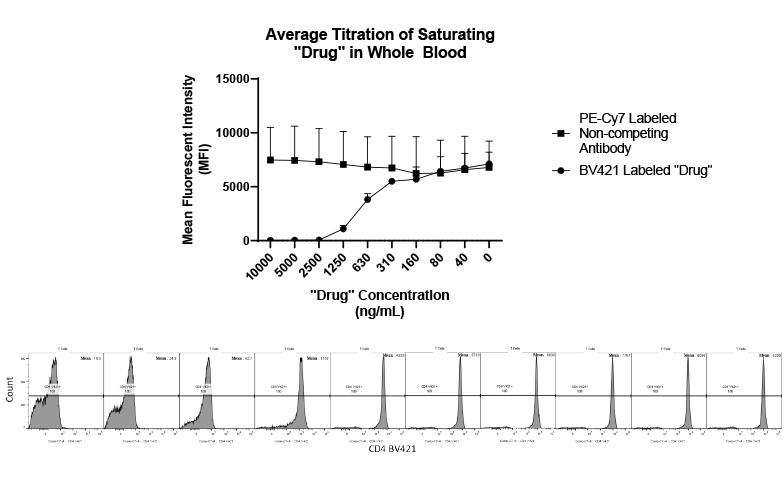
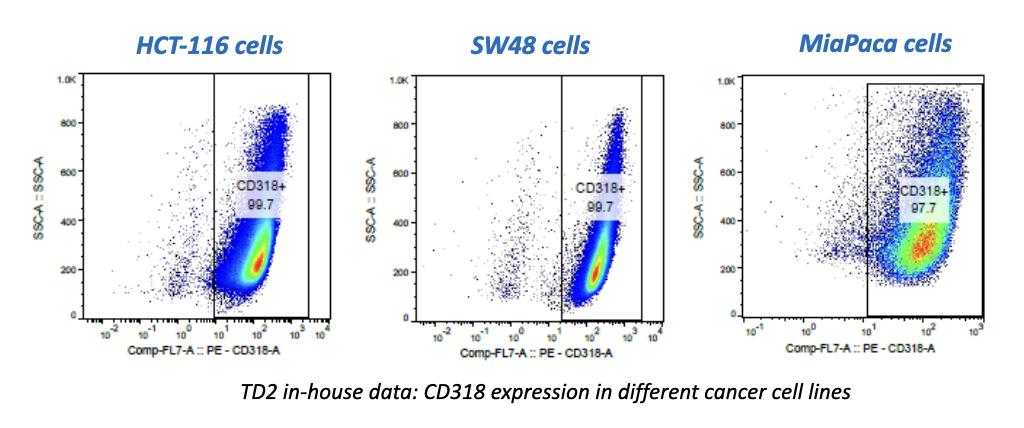
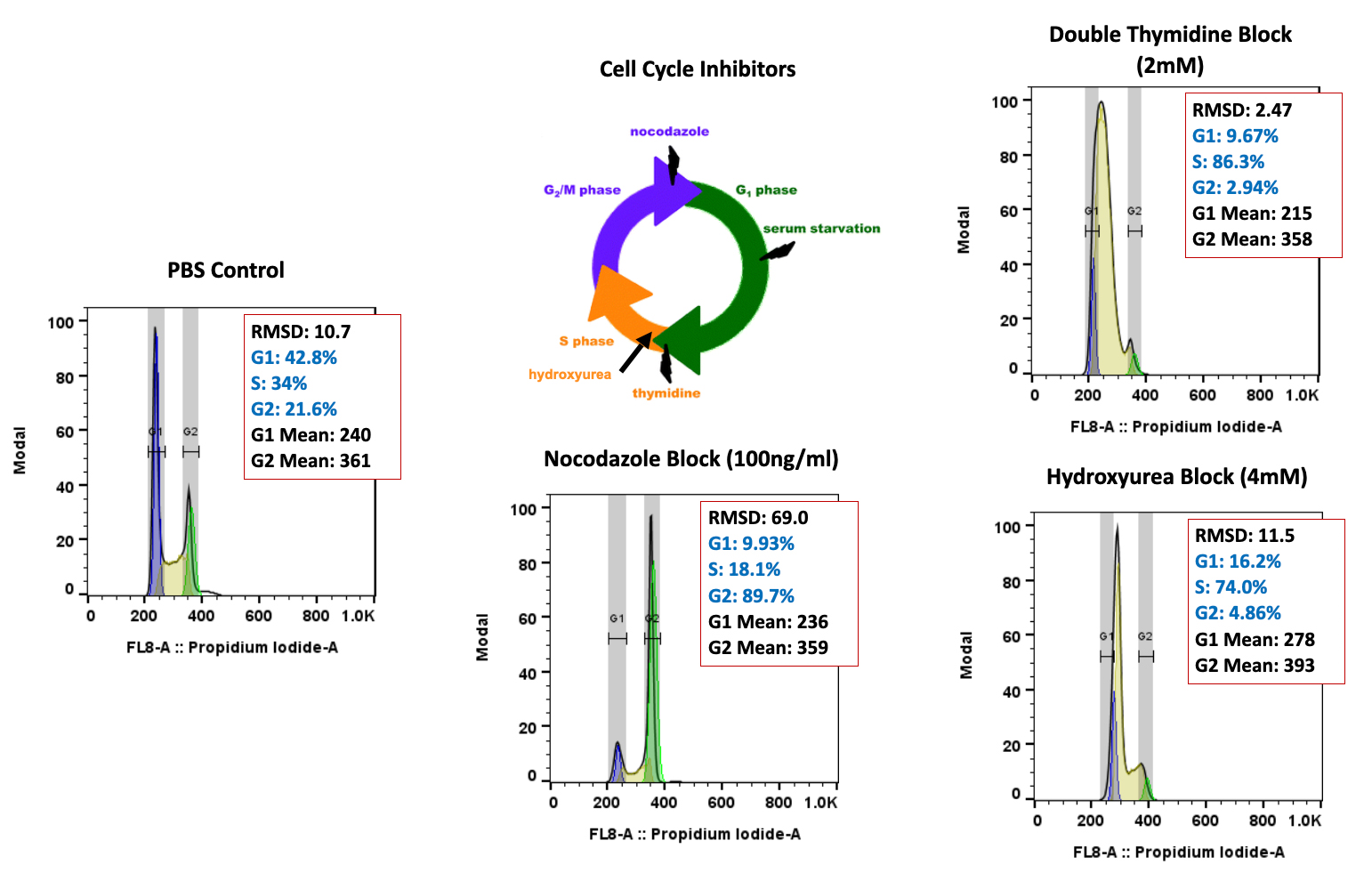
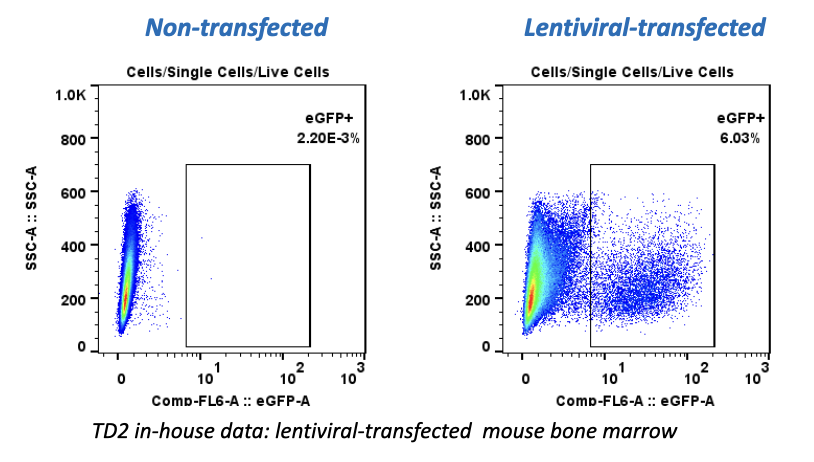
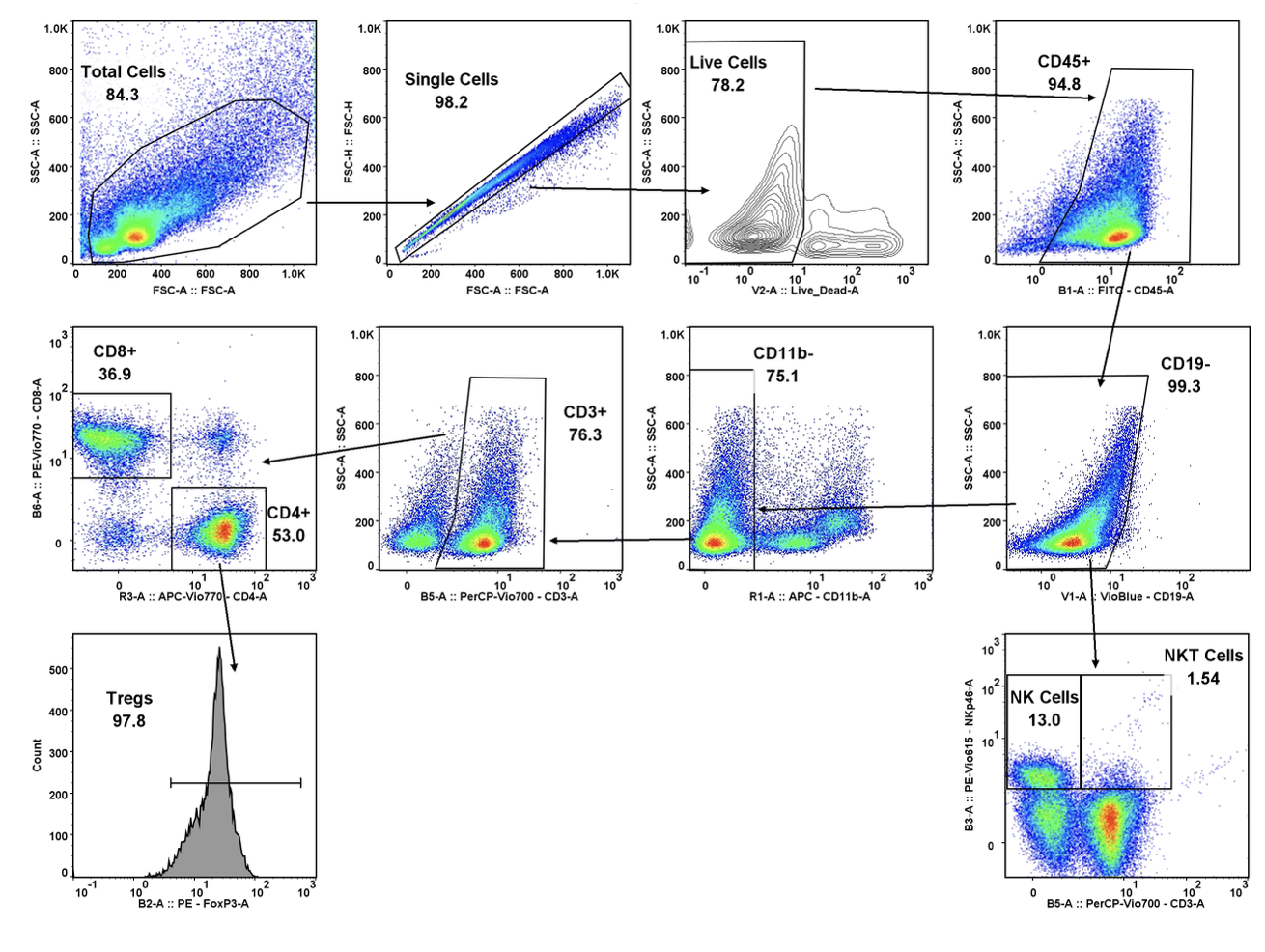
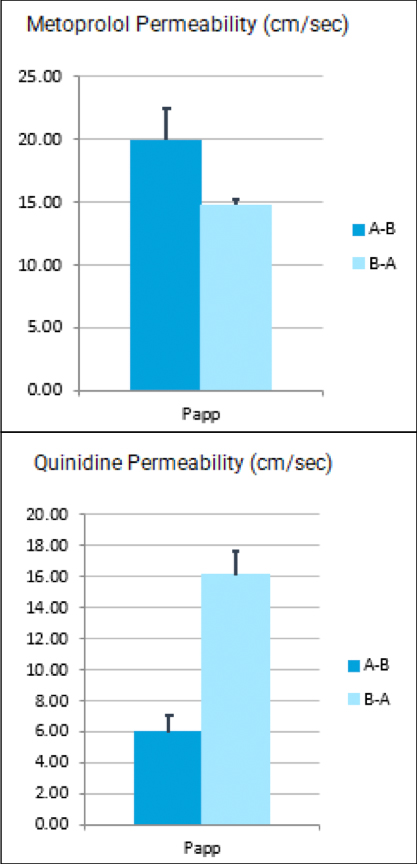
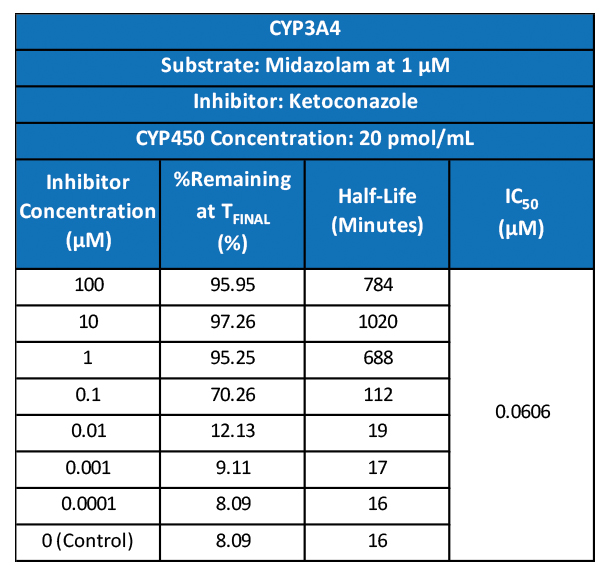
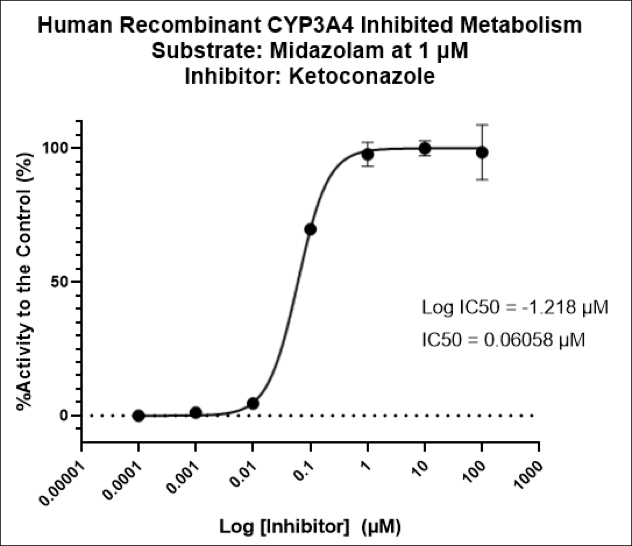
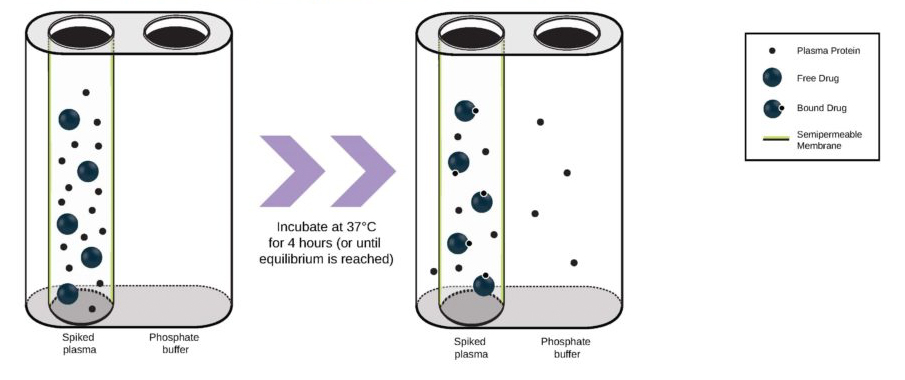
Regulatory
Sometimes, the U.S. FDA is criticized as incapable in comparison with its European counterpart, the EMEA. Particularly this criticism is commonly found in the field of oncology, where highly sick patients have little options for treatment. But now, the FDA is getting their hands on essential therapeutic medications faster than their European counterpart. The latest...
There are two categories for INDs: commercial and research. The main difference is who submits the application to FDA and the intended purpose of...
Types of IND Applications A potential new drug needs IND approval to enter the clinical investigation part of development. IND approval must be...